§ IV. FERMENTATION OF DEXTRO-TARTRATE OF LIME〖See PASTEUR, Comptes rendus de l'Académie des Sciences, vol. lvi., p. 416.〗
TARTRATE of lime, in spite of its insolubility in water, is capable of complete fermentation in a mineral medium.
If we put some pure tartrate of lime, in the form of a granulated, crystalline powder, into pure water, together with some sulphate of ammonia and phosphates of potassium and magnesium, in very small proportions, a spontaneous fermentation will take place in the deposit in the course of a few days, although no germs of ferment have been added. A living, organized ferment, of the vibrionic type, filiform, with tortuous motions, and often of immense length, forms spontaneously by the development of some germs derived in some way from the inevitable particles of dust floating in the air or resting on the surface of the vessels or material which we employ. The germs of the vibrios concerned in putrefaction are diffused around us on every side, and, in all probability, it is one or more of these germs that develop in the medium in question. In this way they effect the decomposition of the tartrate, from which they must necessarily obtain the carbon of their food without which they cannot exist, while the nitrogen is furnished by the ammonia of the ammonical salt, the mineral principles by the phosphate of potassium and magnesium, and the sulphur by the sulphate of ammonia. How strange to see organization, life, and motion orginating under such conditions! Stranger still to think that this organization, life, and motion are effected without the participation of free oxygen. Once the germ gets a primary impulse on its living career by access of oxygen, it goes on reproducing indefinitely, absolutely without atmospheric air. Here then we have a fact which it is important to establish beyond the possibility of doubt, that we may prove that yeast is not the only organized ferment able to live and multiply when out of the influence of free oxygen.
Into a flask, like that represented in FIG. 9, of 2.5 litres (about four pints) in capacity, we put:
Pure, crystallized, neutral tartrate of lime……………… 100 grammesPhosphate of ammonia……………………1 ““ magnesium……………………1 ““ potassium……………………0.5 “Sulphate of ammonia…………………………0.5 “
(1 gramme = 15.43 grains)
To this we added pure distilled water, so as entirely to fill the flask.
In order to expel all the air dissolved in the water and adhering to the solid substances, we first placed our flask in a bath of chloride of calcium in a large cylindrical white iron pot set over a flame. The exit-tube of the flask was plunged in a test tube of Bohemian glass three-quarters full of distilled water, and also heated by a flame. We boiled the liquids in the flask and test-tube for a sufficient time to expel all the air contained in them. We then withdrew the heat from under the test-tube, and immediately afterwards covered the water which it contained with a layer of oil and then permitted the whole apparatus to cool down.
Next day we applied a finger to the open extremity of the exit-tube, which we then plunged in a vessel of mercury. In this particular experiment which we are describing, we permitted the flask to remain in this state for a fortnight. It might have remained there for a century without ever manifesting the least sign of fermentation, the fermentation of the tartrate being a consequence of life, and life after boiling no longer existed in the flask. When it was evident that the contents of the flask were perfectly inert, we impregnated them rapidly, as follows: all the liquid contained in the exit-tube was removed by means of a fine caoutchouc tube, and replaced by about 1 c. (about 17 minims) of liquid and deposit from another flask, similar to the one we have just described, but which had been fermenting spontaneously for twelve days; we lost no time in refilling completely the exit-tube with water which had been first boiled and then cooled down in carbonic acid gas. This operation lasted only a few minutes. The exit-tube was again plunged under mercury. Subsequently the tube was not moved from under the mercury, and as it formed part of the flask, and there was neither cork nor india-rubber, any introduction of air was consequently impossible. The small quantity of air introduced during the impregnation was insignificant and it might even be shown that it injured rather than assisted the growth of the organisms, inasmuch as these consisted of adult individuals which had lived without air and might be liable to be damaged or even destroyed by it. Be this as it may, in a subsequent experiment we shall find the possibility removed of any aeration taking place in this way, however infinitesimal, so that no doubts may linger on this subject.
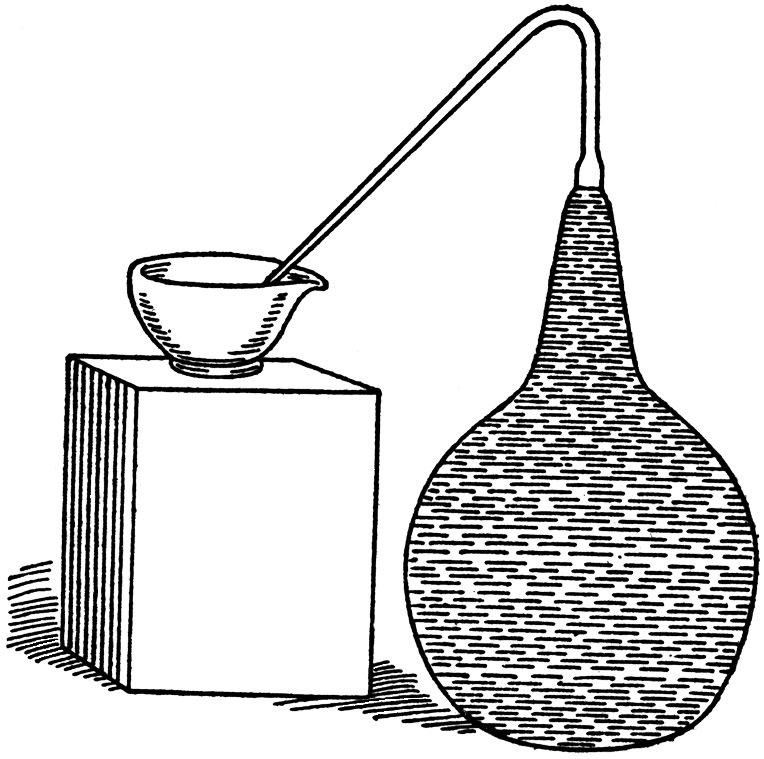
FIG. 9
The following days the organisms multiplied, the deposit of tartrate gradually disappeared, and a sensible ferment action was manifest on the surface, and throughout the bulk of the liquid. The deposit seemed lifted up in places, and was covered with a layer of dark-grey colour, puffed up, and having an organic and gelatinous appearance. For several days, in spite of this action in the deposit, we detected no disengagement of gas, except when the flask was slightly shaken, in which case rather large bubbles adhering to the deposit rose, carrying with them some solid particles, which quickly fell back again, whilst the bubbles diminished in size as they rose, from being partially taken into solution, in consequence of the liquid not being saturated. The smallest bubbles had even time to dissolve completely before they could reach the surface of the liquid. In course of time the liquid was saturated, and the tartrate was gradually displaced by mammillated crusts, or clear, transparent crystals of carbonate of lime at the bottom and on the sides of the vessel.
The impregnation took place on February 10th, and on March 15th the liquid was nearly saturated. The bubbles then began to lodge in the bent part of the exit-tube, at the top of the flask. A glass measuring-tube containing mercury was now placed with its open end over the point of the exit-tube under the mercury in the trough, so that no bubble might escape. A steady evolution of gas went on from the 17th to the 18th, 17.4 cc. (1.06 cubic inches) having been collected. This was proved to be nearly absolutely pure carbonic acid, as indeed might have been suspected from the fact that the evolution did not begin before a distinct saturation of the liquid was observed.〖Carbonic acid being considerably more soluble than other gases possible under the circumstances.—ED.〗
The liquid, which was turbid on the day after its impregnation, had, in spite of the liberation of gas, again become so transparent that we could read our handwriting through the body of the flask. Notwithstanding this, there was still a very active operation going on in the deposit, but it was confined to that spot. Indeed, the swarming vibrios were bound to remain there, the tartrate of lime being still more insoluble in water saturated with carbonate of lime than it is in pure water. A supply of carbonaceous food, at all events, was absolutely wanting in the bulk of the liquid. Every day we continued to collect and analyze the total amount of gas disengaged. To the very last it was composed of pure carbonic acid gas. Only during the first few days did the absorption by the concentrated potash leave a very minute residue. By April 26th all liberation of gas had ceased, the last bubbles having risen in the course of April 23rd. The flask had been all the time in the oven, at a temperature between 25° C. and 28° C. (77° F. and 83° F.). The total volume of gas collected was 2.135 litres (130.2 cubic inches). To obtain the whole volume of gas formed we had to add to this what was held in the liquid in the state of acid carbonate of lime. To determine this we poured a portion of the liquid from the flask into another flask of similar shape, but smaller, up to the gaugemark on the neck.〖We had to avoid filling the small flask completely, for fear of causing some of the liquid to pass on to the surface of the mercury in the measuring tube. The liquid condensed by boiling forms pure water, the solvent affinity of which for carbonic acid, at the temperature we employ, is well known.〗 This smaller flask had been previously filled with carbonic acid. The carbonic acid of the fermented liquid was then expelled by means of heat, and collected over mercury. In this way we found a volume of 8.322 litres (508 cubic inches) of gas in solution, which, added to the 2.135 litres, gave a total of 10.457 litres (638.2 cubic inches) at 20° and 760 mm., which, calculated to 0° C. and 760 mm. atmospheric pressure (32° F. and 30 inches) gave a weight of 19.700 grammes (302.2 grains) of carbonic acid.
Exactly half of the lime in the tartrate employed got used up in the soluble salts formed during fermentation; the other half was partly precipitated in the form of carbonate of lime, partly dissolved in the liquid by the carbonic acid. The soluble salts seemed to us to be a mixture or combination of 1 equivalent of metacetate of lime, with 2 equivalents of the acetate, for every 10 equivalents of carbonic acid produced, the whole corresponding to the fermentation of 3 equivalents of neutral tartrate of lime.〖The following is a curious consequence of these numbers and of the nature of the products of this fermentation. The carbonic acid liberated being quite pure, especially when the liquid has been boiled to expel all air from the flask, and capable of perfect solution, it follows that the volume of liquid being sufficient and the weight of tartrate suitably chosen—we may set aside tartrate of lime in an insoluble, crystalline powder, along with phosphates at the bottom of a closed vessel full of water, and find soon afterwards in their place carbonate of lime, and in the liquid soluble salts of lime, with a mass of organic matter at the bottom, without any liberation of gas or appearance of fermentation ever taking place, except as far as the vital action and transformation in the tartrate are concerned. It is easy to calculate that a vessel or flask of five litres (rather more than a gallon) would be large enough for the accomplishment of this remarkable and singularly quiet transformation, in the case of 50 grammes (767 grains) of tartrate of lime.〗 This point, however, is worthy of being studied with greater care: the present statement of the nature of the products formed is given with all reserve. For our point, indeed, the matter is of little importance, since the equation of the fermentation does not concern us.
After the completion of fermentation there was not a trace of tartrate of lime remaining at the bottom of the vessel: it had disappeared gradually as it got broken up into the different products of fermentation, and its place was taken by some crystallized carbonate of lime—the excess, namely, which had been unable to dissolve by the action of the carbonic acid. Associated, moreover, with this carbonate of lime there was a quantity of some kind of animal matter, which, under the microscope, appeared to be composed of masses of granules mixed with very fine filaments of varying lengths, studded with minute dots, and presenting all the characteristics of a nitrogenous organic substance.〖We treated the whole deposit with dilute hydrochloric acid, which dissolved the carbonate of lime, and the insoluble phosphates of calcium and magnesium; afterwards filtering the liquid through a weighed filter paper. Dried at 100° C. (212° F.), the weight of the organic matter thus obtained was 0.54 gramme (8.3 grains), which was rather more than 1/200th of the weight of fermentable matter.〗 That this was really the ferment is evident enough from all that we have already said. To convince ourselves more thoroughly of the fact, and at the same time to enable us to observe the mode of activity of the organism, we instituted the following supplementary observation. Side by side with the experiment just described, we conducted a similar one, which we intermitted after the fermentation was some-what advanced, and about half of the tartrate dissolved. Breaking off with a file the exit-tube at the point where the neck began to narrow off, we took some of the deposit from the bottom by means of a long straight piece of tubing, in order to bring it under microscopical examination. We found it to consist of a host of long filaments of extreme tenuity, their diameter being about 1/1000th of a millimetre (0.000039 in.); their length varied, in some cases being as much as 1/20th of a millimetre (0.0019 in.). A crowd of these long vibrios were to be seen creeping slowly along, with a sinuous movement, showing three, four, or even five flexures. The filaments that were at rest had the same aspect as these last, with the exception that they appeared punctuate, as though composed of a series of granules arranged in irregular order. No doubt these were vibrios in which vital action had ceased, exhausted specimens which we may compare with the old granular ferment of beer, whilst those in motion may be compared with young and vigorous yeast. The absence of movement in the former seems to prove that this view is correct. Both kinds showed a tendency to form clusters, the compactness of which impeded the movements of those which were in motion. Moreover, it was noticeable that the masses of these latter rested on tartrate not yet dissolved, whilst the granular clusters of the others rested directly on the glass, at the bottom of the flask, as if, having decomposed the tartrate, the only carbonaceous food at their disposal, they had then died on the spot where we captured them, from inability to escape, precisely in consequence of that state of entanglement which they combined to form, during the period of their active development. Besides these we observed vibrios of the same diameter, but of much smaller length, whirling round with great rapidity, and darting backwards and forwards; these were probably identical with the longer ones, and possessed greater freedom of movement, no doubt in consequence of their shortness. Not one of these vibrios could be found throughout the mass of the liquid.
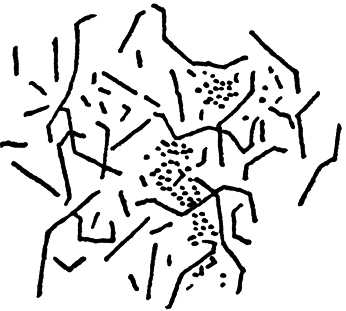
FIG. 10
We may remark that as there was a somewhat putrid odour from the deposit in which the vibrios swarmed, the action must have been one of reduction, and no doubt to this fact was due the greyish coloration of the deposit. We suppose that the substances employed, however pure, always contain some trace of iron, which becomes converted into the sulphide, the black colour of which would modify the originally white deposit of insoluble tartrate and phosphate.
But what is the nature of these vibrios? We have already said that we believe that they are nothing but the ordinary vibrios of putrefaction, reduced to a state of extreme tenuity by the special conditions of nutrition involved in the fermentable medium used; in a word, we think that the fermentation in question might be called putrefaction of tartrate of lime. It would be easy enough to determine this point by growing the vibrios of such fermentation in media adapted to the production of the ordinary forms of vibrio; but this is an experiment which we have not ourselves tried.
One word more on the subject of these curious beings. In a great many of them there appears to be something like a clear spot, a kind of bead, at one of their extremities. This is an illusion arising from the fact that the extremity of these vibrios is curved, hanging downwards, thus causing a greater refraction at that particular point, and leading us to think that the diameter is greater at that extremity. We may easily undeceive ourselves if we watch the movements of the vibrio, when we will readily recognize the bend, especially as it is brought into the vertical plane passing over the rest of the filament. In this way we will see the bright spot, the head, disappear, and then reappear.
The chief inference that it concerns us to draw from the preceding facts is one which cannot admit of doubt, and which we need not insist on any further—namely that vibrios, as met with in the fermentation of neutral tartrate of lime, are able to live and multiply when entirely deprived of air.