§ I. ON THE RELATIONS EXISTING BETWEEN OXYGEN AND YEAST
IT is characteristic of science to reduce incessantly the number of unexplained phenomena. It is observed, for instance, that fleshy fruits are not liable to fermentation so long as their epidermis remains uninjured. On the other hand, they ferment very readily when they are piled up in heaps more or less open, and immersed in their saccharine juice. The mass becomes heated and swells; carbonic acid gas is disengaged, and the sugar disappears and is replaced by alcohol. Now, as to the question of the origin of these spontaneous phenomena, so remarkable in character as well as usefulness for man's service, modern knowledge has taught us that fermentation is the consequence of a development of vegetable cells the germs of which do not exist in the saccharine juices within fruits; that many varieties of these cellular plants exist, each giving rise to its own particular fermentation. The principal products of these various fermentations, although resembling each other in their nature, differ in their relative proportions and in the accessory substances that accompany them, a fact which alone is sufficient to account for wide differences in the quality and commercial value of alcoholic beverages.
Now that the discovery of ferments and their living nature, and our knowledge of their origin, may have solved the mystery of the spontaneous appearance of fermentations in natural saccharine juices, we may ask whether we must still regard the reactions that occur in these fermentations as phenomena inexplicable by the ordinary laws of chemistry. We can readily see that fermentations occupy a special place in the series of chemical and biological phenomena. What gives to fermentations certain exceptional characters of which we are only now beginning to suspect the causes, is the mode of life in the minute plants designated under the generic name of ferments, a mode of life which is essentially different from that in other vegetables, and from which result phenomena equally exceptional throughout the whole range of the chemistry of living beings.
The least reflection will suffice to convince us that the alcoholic ferments must possess the faculty of vegetating and performing their functions out of contact with air. Let us consider, for instance, the method of vintage practised in the Jura. The bunches are laid at the foot of the vine in a large tub, and the grapes there stripped from them. When the grapes, some of which are uninjured, others bruised, and all moistened by the juice issuing from the latter, fill the tub—where they form what is called the vintage—they are conveyed in barrels to large vessels fixed in cellars of a considerable depth. These vessels are not filled to more than three-quarters of their capacity. Fermentation soon takes place in them, and the carbonic acid gas finds escape through the bunghole, the diameter of which, in the case of the largest vessels, is not more than ten or twelve centimetres (about four inches). The wine is not drawn off before the end of two or three months. In this way it seems highly probable that the yeast which produces the wine under such conditions must have developed, to a great extent at least, out of contact with oxygen. No doubt oxygen is not entirely absent from the first; nay, its limited presence is even a necessity to the manifestation of the phenomena which follow. The grapes are stripped from the bunch in contact with air, and the must which drops from the wounded fruit takes a little of this gas into solution. This small quantity of air so introduced into the must, at the commencement of operations, plays a most indispensable part, it being from the presence of this that the spores of ferments which are spread over the surface of the grapes and the woody part of the bunches derive the power of starting their vital phenomena.〖It has been remarked in practice that fermentation is facilitated by leaving the grapes on the bunches. The reason of this has not yet been discovered. Still we have no doubt that it may be attributed, principally, to the fact that the interstices between the grapes, and the spaces which the bunch leaves throughout, considerably increase the volume of air placed at the service of the germs of ferment.〗 This air, however, especially when the grapes have been stripped from the bunches, is in such small proportion, and that which is in contact with the liquid mass is so promptly expelled by the carbonic acid gas, which is evolved as soon as a little yeast has formed, that it will readily be admitted that most of the yeast is produced apart from the influence of oxygen, whether free or in solution. We shall revert to this fact, which is of great importance. At present we are only concerned in pointing out that, from the mere knowledge of the practices of certain localities, we are induced to believe that the cells of yeast, after they have developed from their spores, continue to live and multiply without the intervention of oxygen, and that the alcoholic ferments have a mode of life which is probably quite exceptional, since it is not generally met with in other species, vegetable or animal.
Another equally exceptional characteristic of yeast and fermentation in general consists in the small proportion which the yeast that forms bears to the sugar that decomposes. In all other known beings the weight of nutritive matter assimilated corresponds with the weight of food used up, any difference that may exist being comparatively small. The life of yeast is entirely different. For a certain weight of yeast formed, we may have ten times, twenty times, a hundred times as much sugar, or even more decomposed, as we shall experimentally prove by-and-by; that is to say, that whilst the proportion varies in a precise manner, according to conditions which we shall have occasion to specify, it is also greatly out of proportion to the weight of the yeast. We repeat, the life of no other being, under its normal physiological conditions, can show anything similar. The alcoholic ferments, therefore, present themselves to us as plants which possess at least two singular properties: they can live without air, that is without oxygen, and they can cause decomposition to an amount which, though variable, yet, as estimated by weight of product formed, is out of all proportion to the weight of their own substance. These are facts of so great importance, and so intimately connected with the theory of fermentation, that it is indispensable to endeavour to establish them experimentally, with all the exactness of which they will admit.
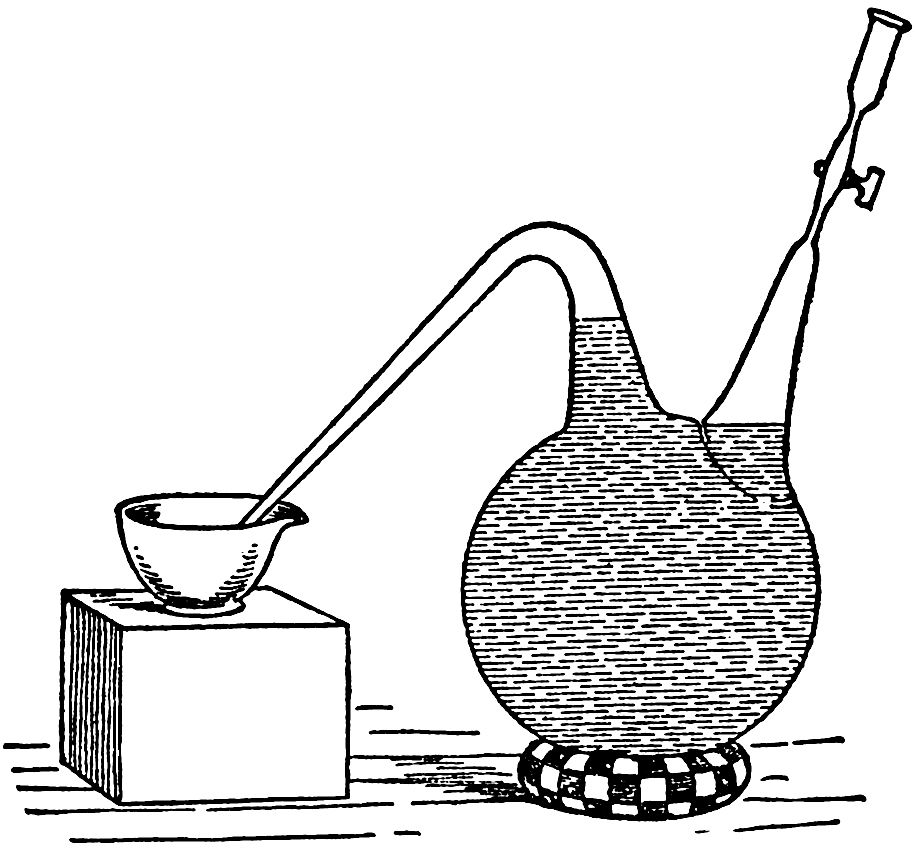
FIG. 1
The question before us is whether yeast is in reality an anaërobian〖Capable of living without free oxygen—a term invented by Pasteur.—ED.〗 plant, and what quantities of sugar it may cause to ferment, under the various conditions under which we cause it to act.
The following experiments were undertaken to solve this double problem:—We took a double-necked flask, of three litres (five pints) capacity, one of the tubes being curved and forming an escape for the gas; the other one, on the right hand side (FIG. 1), being furnished with a glass tap. We filled this flask with pure yeast water, sweetened with 5 per cent. of sugar candy, the flask being so full that there was not the least trace of air remaining above the tap or in the escape tube; this artificial wort had, however, been itself aerated. The curved tube was plunged in a porcelain vessel full of mercury, resting on a firm support. In the small cylindrical funnel above the tap, the capacity of which was from 10 cc. to 15 cc. (about half a fluid ounce) we caused to ferment, at a temperature of 20° or 25° C. (about 75° F.), five or six cubic centimetres of the saccharine liquid, by means of a trace of yeast, which multiplied rapidly, causing fermentation, and forming a slight deposit of yeast at the bottom of the funnel above the tap. We then opened the tap, and some of the liquid in the funnel entered the flask, carrying with it the small deposit of yeast, which was sufficient to impregnate the saccharine liquid contained in the flask. In this manner it is possible to introduce as small a quantity of yeast as we wish, a quantity the weight of which, we may say, is hardly appreciable. The yeast sown multiplies rapidly and produces fermentation, the carbonic gas from which is expelled into the mercury. In less than twelve days all the sugar had disappeared, and the fermentation had finished. There was a sensible deposit of yeast adhering to the sides of the flask; collected and dried it weighed 2.25 grammes (34 grains). It is evident that in this experiment the total amount of yeast formed, if it required oxygen to enable it to live, could not have absorbed, at most, more than the volume which was originally held in solution in the saccharine liquid, when that was exposed to the air before being introduced into the flask.
Some exact experiments conducted by M. Raulin in our laboratory have established the fact that saccharine worts, like water, soon become saturated when shaken briskly with an excess of air, and alsothat they always take into solution a little less air than saturatedpure water contains under the same conditions of temperature and pressure. At a temperature of 25° C. (77° F.), therefore, if we adopt the coefficient of the solubility of oxygen in water given in Bunsen's tables, we find that 1 litre (1 3/4 pints) of water saturated with air contains 5.5 cc. (0.3 cubic inch) of oxygen. The three litres of yeast-water in the flask, supposing it to have been saturated, contains less than 16.5 cc. (1 cubic inch) of oxygen, or, in weight, less than 23 milligrammes (0.35 grains). This was the maximum amount of oxygen, supposing the greatest possible quantity to have been absorbed, that was required by the yeast formed in the fermentation of 150 grammes (4.8 Troy ounces) of sugar. We shall better understand the significance of this result later on. Let us repeat the foregoing experiment, but under altered conditions. Let us fill, as before, our flask with sweetened yeast-water, but let this first be boiled, so as to expel all the air it contains. To effect this we arrange our apparatus as represented in the accompanying sketch. (FIG. 2.)
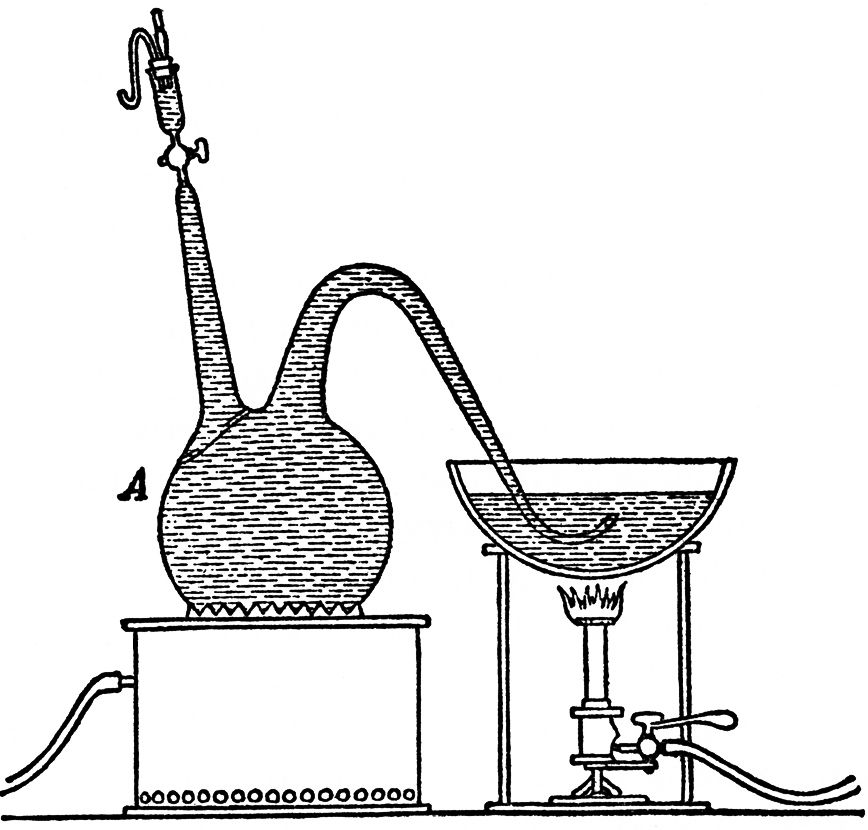
FIG. 2
We place our flask, A, on a tripod above a gas flame, and in place of the vessel of mercury substitute a porcelain dish, under which we can put a gas flame, and which contains some fermentable, saccharine liquid, similar to that with which the flask is filled. We boil the liquid in the flask and that in the basin simultaneously, and then let them cool down together, so that as the liquid in the flask cools some of the liquid is sucked from the basin into the flask. From a trial experiment which we conducted, determining the quantity of oxygen that remained in solution in the liquid after cooling, according to M. Schützenberger's valuable method, by means of hydrosulphite of soda, (NaHSO2, now called Sodium hyposulphite.—D. C. R.) we found that the three litres in the flask, treated as we have described, contained less than one milligramme (0.015 grain) of oxygen. At the same time we conducted another experiment, by way of comparison (FIG. 3). We took a flask, B, of larger capacity than the former one, which we filled about half with the same volume as before of a saccharine liquid of identically the same composition. This liquid had been previously freed from alterative germs by boiling. In the funnel surmounting A, we put a few cubic centimetres of saccharine liquid in a state of fermentation, and when this small quantity of liquid was in full fermentation, and the yeast in it was young and vigorous, we opened the tap, closing it again immediately, so that a little of the liquid and yeast still remained in the funnel. By this means we caused the liquid in A to ferment. We also impregnated the liquid in B with some yeast taken from the funnel of A. We then replaced the porcelain dish in which the curved escape tube of A had been plunged, by a vessel filled with mercury. The following is a description of two of these comparative fermentations and the results they gave.
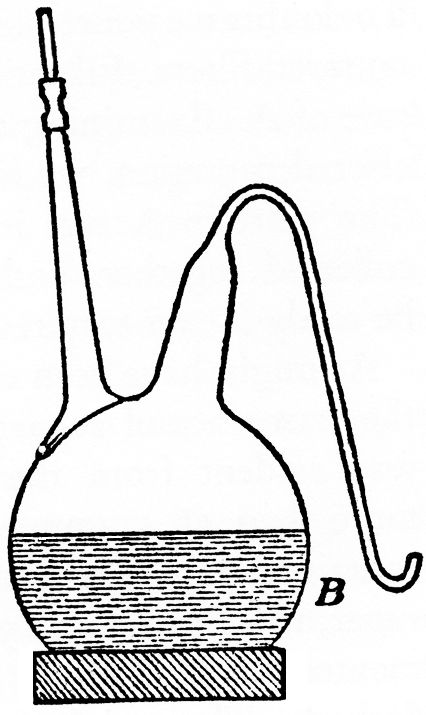
FIG. 3
The fermentable liquid was composed of yeast-water sweetened with 5 per cent. of sugar-candy; the ferment employed was sacchormyces pastorianus.
The impregnation took place on January 20th. The flasks were placed in an oven at 25° (77° F.).
Flask A, without air.
January 21st.—Fermentation commenced; a little frothy liquid issued from the escape tube and covered the mercury.
The following days, fermentation was active. Examining the yeast mixed with the froth that was expelled into the mercury by the evolution of carbonic acid gas, we find that it was very fine, young, and actively budding.
February 3rd.—Fermentation still continued, showing itself by a number of little bubbles rising from the bottom of the liquid, which had settled bright. The yeast was at the bottom in the form of a deposit.
February 7th.—Fermentation still continued, but very languidly.
February 9th.—A very languid fermentation still went on, discernible in little bubbles rising from the bottom of the flask.
Flask B, with air.
January 21st.—A sensible development of yeast.
The following days, fermentation was active, and there was an abundant froth on the surface of the liquid.
February 1st.—All symptoms of fermentation had ceased.
As the fermentation in A would have continued a long time, being so very languid, and as that in B had been finished for several days, we brought to a close our two experiments on February 9th. To do this we poured off the liquids in A and B, collecting the yeasts on tared filters. Filtration was an easy matter, more especially in the case of A. Examining the yeasts under the microscope, immediately after decantation, we found that both of them remained very pure. The yeast in A was in little clusters, the globules of which were collected together, and appeared by their well-defined borders to be ready for an easy revival in contact with air.
As might have been expected, the liquid in flask B did not contain the least trace of sugar; that in the flask A still contained some, as was evident from the non-completion of fermentation, but not more than 4.6 grammes (71 grains). Now, as each flask originally contained three litres of liquid holding in solution 5 per cent. of sugar, it follows that 150 grammes (2,310 grains) of sugar had fermented in the flask B, and 145.4 grammes (2,239.2 grains) in the flask A. The weights of yeast after drying at 100° C. (212° F.) were—
For the flask B, with air …… 1,970 grammes (30.4 grains).
For the flask A, without air …… 1,368 grammes.〖This appears to be a misprint for 1.638 grammes=25.3 grains.—D. C. R.〗
The proportions were 1 of yeast to 76 of fermented sugar in the first case, and 1 of yeast to 89 of fermented sugar in the second.
From these facts the following consequences may be deduced:
1. The fermentable liquid (flask B), which since it had been in contact with air, necessarily held air in solution, although not to the point of saturation, inasmuch as it had been once boiled to free it from all foreign germs, furnished a weight of yeast sensibly greater than that yielded by the liquid which contained no air at all (flask A) or, at least, which could only have contained an exceedingly minute quantity.
2. This same slightly aerated fermentable liquid fermented much more rapidly than the other. In eight or ten days it contained no more sugar; while the other, after twenty days, still contained an appreciable quantity.
Is this last fact to be explained by the greater quantity of yeast formed in B? By no means. At first, when the air has access to the liquid, much yeast is formed and little sugar disappears, as we shall prove immediately; nevertheless the yeast formed in contact with the air is more active than the other. Fermentation is correlative first to the development of the globules, and then to the continued life of those globules once formed. The more oxygen these last globules have at their disposal during their formation, the more vigorous, transparent, and turgescent, and, as a consequence of this last quality, the more active they are in decomposing sugar. We shall hereafter revert to these facts.
3. In the airless flask the proportion of yeast to sugar was 1/89; it was only 1/76 in the flask which had air at first.
The proportion that the weight of yeast bears to the weight of the sugar is, therefore, variable, and this variation depends, to a certain extent, upon the presence of air and the possibility of oxygen being absorbed by the yeast. We shall presently show that yeast possesses the power of absorbing that gas and emitting carbonic acid, like ordinary fungi, that even oxygen may be reckoned amongst the number of food-stuffs that may be assimilated by this plant, and that this fixation of oxygen in yeast, as well as the oxidations resulting from it, have the most marked effect on the life of yeast, on the multiplication of its cells, and on their activity as ferments acting upon sugar, whether immediately or afterwards, apart from supplies of oxygen or air.
In the preceding experiment, conducted without the presence of air, there is one circumstance particularly worthy of notice. This experiment succeeds, that is to say, the yeast sown in the medium deprived of oxygen develops, only when this yeast is in a state of great vigour. We have already explained the meaning of this last expression. But we wish now to call attention to a very evident fact in connection with this point. We impregnate a fermentable liquid; yeast develops and fermentation appears. This lasts for several days and then ceases. Let us suppose that, from the day when fermentation first appears in the production of a minute froth, which gradually increases until it whitens the surface of the liquid, we take, every twenty-four hours, or at longer intervals, a trace of the yeast deposited on the bottom of the vessel and use it for starting fresh fermentations. Conducting these fermentations all under precisely the same conditions of temperature, character and volume of liquid, let us continue this for a prolonged time, even after the original fermentation is finished. We shall have no difficulty in seeing that the first signs of action in each of our series of second fermentations appear always later and later in proportion to the length of time that has elapsed from the commencement of the original fermentation. In other words, the time necessary for the development of the germs and the production of that amount of yeast sufficient to cause the first appearance of fermentation varies with the state of the impregnating cells, and is longer in proportion as the cells are further removed from the period of their formation. It is essential, in experiments of this kind, that the quantities of yeast successively taken should be as nearly as possible equal in weight or volume, since, ceteris paribus, fermentations manifest themselves more quickly the larger the quantity of yeast employed in impregnation.
If we compare under the microscope the appearance and character of the successive quantities of yeast taken, we shall see plainly that the structure of the cells undergoes a progressive change. The first sample which we take, quite at the beginning of the original fermentation, generally gives us cells rather larger than those later on, and possessing a remarkable tenderness. Their walls are exceedingly thin, the consistency and softness of their protoplasm is akin to fluidity, and their granular contents appear in the form of scarcely visible spots. The borders of the cells soon become more marked, a proof that their walls undergo a thickening; their protoplasm also becomes denser, and the granulations more distinct. Cells of the same organ, in the states of infancy and old age, should not differ more than the cells of which we are speaking, taken in their extreme states. The progressive changes in the cells, after they have acquired their normal form and volume, clearly demonstrate the existence of a chemical work of a remarkable intensity, during which their weight increases, although in volume they undergo no sensible change, a fact that we have often characterized as “the continued life of cells already formed.” We may call this work a process of maturation on the part of the cells, almost the same that we see going on in the case of adult beings in general, which continue to live for a long time, even after they have become incapable of reproduction, and long after their volume has become permanently fixed.
This being so, it is evident, we repeat, that, to multiply in a fermentable medium, quite out of contact with oxygen, the cells of yeast must be extremely young, full of life and health, and still under the influence of the vital activity which they owe to the free oxygen which has served to form them, and which they have perhaps stored up for a time. When older, they reproduce themselves with much difficulty when deprived of air, and gradually become more languid; and if they do multiply, it is in strange and monstrous forms. A little older still, they remain absolutely inert in a medium deprived of free oxygen. This is not because they are dead; for in general they may be revived in a marvellous manner in the same liquid if it has been first aerated before they are sown. It would not surprise us to learn that at this point certain preconceived ideas suggest themselves to the mind of an attentive reader on the subject of the causes that may serve to account for such strange phenomena in the life of these beings which our ignorance hides under the expressions of youth and age; this, however, is a subject which we cannot pause to consider here.
At this point we must observe—for it is a matter of great importance—that in the operations of the brewer there is always a time when the yeasts are in this state of vigorous youth of which we have been speaking, acquired under the influence of free oxygen, since all the worts and the yeasts of commerce are necessarily manipulated in contact with air, and so impregnated more or less with oxygen. The yeast immediately seizes upon this gas and acquires a state of freshness and activity, which permits it to live afterwards out of contact with air, and to act as a ferment. Thus, in ordinary brewery practice, we find the yeast already formed in abundance even before the earliest external signs of fermentation have made their appearance. In this first phase of its existence, yeast lives chiefly like an ordinary fungus.
From the same circumstances it is clear that the brewer's fermentations may, speaking quite strictly, last for an indefinite time, in consequence of the unceasing supply of fresh wort, and from the fact, moreover, that the exterior air is constantly being introduced during the work, and that the air contained in the fresh worts keeps up the vital activity of the yeast, as the act of breathing keeps up the vigour and life of cells in all living beings. If the air could not renew itself in any way, the vital activity which the cells originally received, under its influence, would become more and more exhausted, and the fermentation eventually come to an end.
We may recount one of the results obtained in other experiments similar to the last, in which, however, we employed yeast which was still older than that used for our experiment with flask A (FIG. 2), and moreover took still greater precautions to prevent the presence of air. Instead of leaving the flask, as well as the dish, to cool slowly, after having expelled all air by boiling, we permitted the liquid in the dish to continue boiling whilst the flask was being cooled by artificial means; the end of the escape tube was then taken out of the still boiling dish and plunged into the mercury trough. In impregnating the liquid, instead of employing the contents of the small cylindrical funnel whilst still in a state of fermentation, we waited until this was finished. Under these conditions, fermentation was still going on in our flask, after a lapse of three months. We stopped it and found that 0.255 gramme (3.9 grains) of yeast had been formed, and that 45 grammes (693 grains) of sugar had fermented, the ratio between the weights of yeast and sugar being thus 0.255/45=1/176. In this experiment the yeast developed with much difficulty, by reason of the conditions to which it had been subjected. In appearance the cells varied much, some were to be found large, elongated, and of tubular aspect, some seemed very old and were extremely granular, whilst others were more transparent. All of them might be considered abnormal cells.
In such experiments we encounter another difficulty. If the yeast sown in the non-aerated fermentable liquid is in the least degree impure, especially if we use sweetened yeast-water, we may be sure that alcoholic fermentation will soon cease, if, indeed, it ever commences, and that accessory fermentations will go on. The vibrios of butyric fermentation, for instance, will propagate with remarkable facility under these circumstances. Clearly then, the purity of the yeast at the moment of impregnation, and the purity of the liquid in the funnel, are conditions indispensable to success.
To secure the latter of these conditions, we close the funnel, as shown in FIG. 2, by means of a cork pierced with two holes, through one of which a short tube passes, to which a short length of india-rubber tubing provided with a glass stopper is attached; through the other hole a thin curved tube is passed. Thus fitted, the funnel can answer the same purposes as our double-necked flasks. A few cubic centimetres of sweetened yeast-water are put in it and boiled, so that the steam may destroy any germs adhering to the sides; and when cold the liquid is impregnated by means of a trace of pure yeast, introduced through the glass-stoppered tube. If theseprecautions are neglected, it is scarcely possible to secure a successful fermentation in our flasks, because the yeast sown is immediately held in check by a development of anaërobian vibrios. For greater security, we may add to the fermentable liquid, at the moment when it is prepared, a very small quantity of tartaric acid, which will prevent the development of butyric vibrios.
The variation of the ratio between the weight of the yeast and that of the sugar decomposed by it now claims special attention. Side by side with the experiments which we have just described, we conducted a third lot by means of the flask C (FIG. 4), holding 4.7 litres (8 1/2 pints), and fitted up like the usual two-necked flasks, with the object of freeing the fermentable liquid from foreign germs, by boiling it to begin with, so that we might carry on our work under conditions of purity. The volume of yeast-water (containing 5 per cent. of sugar) was only 200 cc. (7 fl. oz.), and consequently, taking into account the capacity of the flask, it formed but a very thin layer at the bottom. On the day after impregnation the deposit of yeast was already considerable, and forty-eight hours afterwards the fermentation was completed. On the third day we collected the yeast after having analyzed the gas contained in the flask. This analysis was easily accomplished by placing the flask in a hot-water bath, whilst the end of the curved tube was plunged under a cylinder of mercury. The gas contained 41.4 per cent. of carbonic acid, and, after the absorption, the remaining air contained:—
Oxygen 19.7
Nitrogen 80.3/100.0
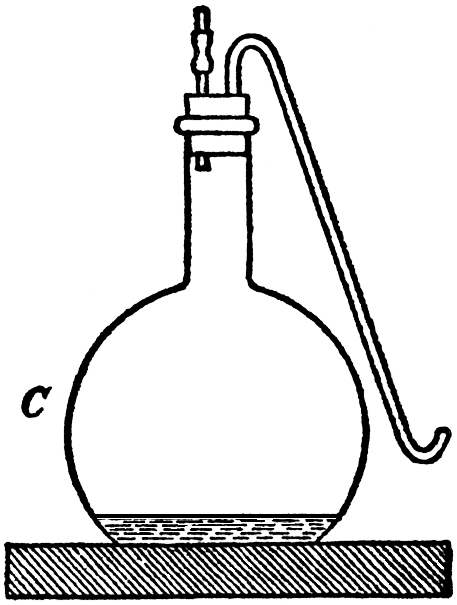
FIG. 4
Taking into consideration the volume of this flask, this shows a minimum of 50 cc. (3.05 cub. in.) of oxygen to have been absorbed by the yeast. The liquid contained no more sugar, and the weight of the yeast, dried at a temperature of 100° C. (212° F.), was 0.44 grammes. The ratio between the weights of yeast and sugar is 0.44/10=1/22.7.〖200 cc. of liquid were used, which, as containing 5 per cent., had in solution 10 grammes of sugar.—D. C. R.〗 On this occasion, where we had increased the quantity of oxygen held in solution, so as to yield itself for assimilation at the beginning and during the earlier developments of the yeast, we found instead of the previous ratio of 1/76 that of 1/23.
The next experiment was to increase the proportion of oxygen to a still greater extent, by rendering the diffusion of gas a more easy matter than in a flask, the air in which is in a state of perfect quiescence. Such a state of matters hinders the supply of oxygen, inasmuch as the carbonic acid, as soon as it is liberated, at once forms an immovable layer on the surface of the liquid, and so separates off the oxygen. To effect the purpose of our present experiment, we used flat basins having glass bottoms and low sides, also of glass, in which the depth of the liquid is not more than a few millimetres (less than 1/4 inch [FIG. 5]). The following is one of our experiments so conducted:—On April 16th, 1860, we sowed a trace of beer yeast (“high” yeast) in 200 cc. (7 fl. oz.) of a saccharine liquid containing 1.720 grammes (26.2 grains) of sugarcandy. From April 18th our yeast was in good condition and well developed. We collected it, after having added to the liquid a few drops of concentrated sulphuric acid, with the object of checking the fermentation to a great extent, and facilitating filtration. The sugar remaining in the filtered liquid, determined by Fehling's solution, showed that 1.04 grammes (16 grains) of sugar had disappeared. The weight of the yeast, dried at 100° C. (212° F.), was 0.127 gramme (2 grains), which gives us the ratio between the weight of the yeast and that of the fermented sugar 0.127/1.04=1/8.1, which is considerably higher than the preceding ones.

FIG. 5
We may still further increase this ratio by making our estimation as soon as possible after the impregnation, or the addition of the ferment. It will be readily understood why yeast, which is composed of cells that bud and subsequently detach themselves from one another, soon forms a deposit at the bottom of the vessels. In consequence of this habit of growth, the cells constantly covering each other prevents the lower layers from having access to the oxygen held in solution in the liquid, which is absorbed by the upper ones. Hence, these which are covered and deprived of this gas act on the sugar without deriving any vital benefit from the oxygen—a circumstance which must tend to diminish the ratio of which we are speaking. Once more repeating the preceding experiment, but stopping it as soon as we think that the weight of yeast formed may be determined by the balance (we find that this may be done twenty-four hours after impregnation with an inappreciable quantity of yeast), in this case the ratio between the weights of yeast and sugar is 0 gr. 024 yeast/0 gr. 098 sugar=1/4. This is the highest ratio we have been able to obtain.
Under these conditions the fermentation of sugar is extremely languid: the ratio obtained is very nearly the same that ordinary fungoid growths would give. The carbonic acid evolved is principally formed by the decompositions which result from the assimilation of atmospheric oxygen. The yeast, therefore, lives and performs its functions after the manner of ordinary fungi: so far it is no longer a ferment, so to say; moreover, we might expect to find it to cease to be a ferment at all if we could only surround each cell separately with all the air that it required. This is what the preceding phenomena teach us; we shall have occasion to compare them later on with others which relate to the vital action exercised on yeast by the sugar of milk.
We may here be permitted to make a digression.
In his work on fermentations, which M. Schützenberger has recently published, the author criticises the deductions that we have drawn from the preceding experiments, and combats the explanation which we have given of the phenomena of fermentation.〖International Science Series, vol. xx, pp. 179-182. London, 1876.—D. C. R.〗 It is an easy matter to show the weak point of M. Schützenberger's reasoning. We determined the power of the ferment by the relation of the weight of sugar decomposed to the weight of the yeast produced. M. Schützenberger asserts that in doing this we lay down a doubtful hypothesis, and he thinks that this power, which he terms fermentative energy, may be estimated more correctly by the quantity of sugar decomposed by the unit-weight of yeast in unit-time; moreover, since our experiments show that yeast is very vigorous when it has a sufficient supply of oxygen, and that, in such a case, it can decompose much sugar in a little time, M. Schützenberger concludes that it must then have great power as a ferment, even greater than when it performs its functions without the aid of air, since under this condition it decomposes sugar very slowly. In short, he is disposed to draw from our observations the very opposite conclusion to that which we arrived at.
M. Schützenberger has failed to notice that the power of a ferment is independent of the time during which it performs its functions. We placed a trace of yeast in one litre of saccharine wort; it propagated, and all the sugar was decomposed. Now, whether the chemical action involved in this decomposition of sugar had required for its completion one day, or one month, or one year, such a factor was of no more importance in this matter than the mechanical labour required to raise a ton of materials from the ground to the top of a house would be affected by the fact that it had taken twelve hours instead of one. The notion of time has nothing to do with the definition of work. M. Schützenberger has not perceived that in introducing the consideration of time into the definition of the power of a ferment, he must introduce at the same time, that of the vital activity of the cells which is independent of their character as a ferment. Apart from the consideration of the relation existing between the weight of fermentable substance decomposed and that of ferment produced, there is no occasion to speak of fermentations or of ferments. The phenomena of fermentation and of ferments have been placed apart from others, precisely because, in certain chemical actions, that ratio has been out of proportion; but the time that these phenomena require for their accomplishment has nothing to do with either their existence proper, or with their power. The cells of a ferment may, under some circumstances, require eight days for revival and propagation, whilst, under other conditions, only a few hours are necessary; so that, if we introduce the notion of time into our estimate of their power of decomposition, we may be led to conclude that in the first case that power was entirely wanting, and that in the second case it was considerable, although all the time we are dealing with the same organism—the identical ferment.
M. Schützenberger is astonished that fermentation can take place in the presence of free oxygen, if, as we suppose, the decomposition of the sugar is the consequence of the nutrition of the yeast, at the expense of the combined oxygen, which yields itself to the ferment. At all events, he argues, fermentation ought to be slower in the presence of free oxygen. But why should it be slower? We have proved that in the presence of oxygen the vital activity of the cells increases, so that, as far as rapidity of action is concerned, its power cannot be diminished. It might, nevertheless, be weakened as a ferment, and this is precisely what happens. Free oxygen imparts to the yeast a vital activity, but at the same time impairs its power as yeast—quâ yeast, inasmuch as under this condition it approaches the state in which it can carry on its vital processes after the manner of an ordinary fungus; the mode of life, that is, in which the ratio between the weight of sugar decomposed and the weight of the new cells produced will be the same as holds generally among organisms which are not ferments. In short, varying our form of expression a little, we may conclude with perfect truth, from the sum total of observed facts, that the yeast which lives in the presence of oxygen and can assimilate as much of that gas as is necessary to its perfect nutrition, ceases absolutely to be a ferment at all. Nevertheless, yeast formed under these conditions and subsequently brought into the presence of sugar, out of the influence of air, would decompose more in a given time than in any other of its states. The reason is that yeast which has formed in contact with air, having the maximum of free oxygen that it can assimilate is fresher and possessed of greater vital activity than that which has been formed without air or with an insufficiency of air. M. Schützenberger would associate this activity with the notion of time in estimating the power of the ferment; but he forgets to notice that yeast can only manifest this maximum of energy under a radical change of its life conditions; by having no more air at its disposal and breathing no more free oxygen. In other words, when its respiratory power becomes null, its fermentative power is at its greatest. M. Schützenberger asserts exactly the opposite (p. 151 of his work—Paris. 1875),〖Page 182, English edition.〗 and so gratuitously places himself in opposition to facts.
FIG. 6
In presence of abundant air supply, yeast vegetates with extraordinary activity. We see this in the weight of new yeast, comparatively large, that may be formed in the course of a few hours. The microscope still more clearly shows this activity in the rapidity of budding, and the fresh and active appearance of all the cells. FIG. 6 represents the yeast of our last experiment at the moment when we stopped the fermentation. Nothing has been taken from imagination, all the groups have been faithfully sketched as they were.〖This figure is on a scale of 300 diameters, most of the figures in this work being of 400 diameters.〗
In passing it is of interest to note how promptly the preceding results were turned to good account practically. In well-managed distilleries, the custom of aerating the wort and the juices to render them more adapted to fermentation, has been introduced. The molasses mixed with water is permitted to run in thin threads through the air at the moment when the yeast is added. Manufactories have been erected in which the manufacture of yeast is almost exclusively carried on. The saccharine worts, after the addition of yeast, are left to themselves, in contact with air, in shallow vats of large superficial area, realizing thus on an immense scale the conditions of the experiments which we undertook in 1861, and which we have already described in determining the rapid and easy multiplication of yeast in contact with air.
The next experiment was to determine the volume of oxygen absorbed by a known quantity of yeast, the yeast living in contact with air, and under such conditions that the absorption of air was comparatively easy and abundant.
With this object we repeated the experiment that we performed with the large-bottomed flask (FIG. 4), employing a vessel shaped like Fig. B (FIG. 7), which is, in point of fact, the flask A with its neck drawn out and closed in a flame, after the introduction of a thin layer of some saccharine juice impregnated with a trace of pure yeast. The following are the data and results of an experiment of this kind.
FIG. 7
We employed 60 cc. (about 2 fluid ounces) of yeast-water, sweetened with two per cent. of sugar and impregnated with a trace of yeast. After having subjected our vessel to a temperature of 25° C. (77° F.) in an oven for fifteen hours, the drawn-out point was brought under an inverted jar filled with mercury and the point broken off. A portion of the gas escaped and was collected in the jar. For 25 cc. of this gas we found, after absorption by potash, 20.6, and after absorption by pyrogallic acid, 17.3. Taking into account the volume which remained free in the flask, which held 315 cc., there was a total absorption of 14.5 cc. (0.88 cub. in.) of oxygen.〖It may be useful for the non-scientific reader to put it thus: that the 25 cc. which escaped, being a fair sample of the whole gas in the flask, and containing (1) 25—20.6=4.4 cc., absorbed by potash and therefore due to carbonic acid, and (2) 20.6—17.3=3.3 cc., absorbed by pyrogallate, and therefore due to oxygen, and the remaining 17.3 cc. being nitrogen, the whole gas in the flask, which has a capacity of 312 cc., will contain oxygen in the above proportion and therefore its amount may be determined, provided we know the total gas in the flask before opening. On the other hand we know that air normally contains approximately, 1-5 its volume of oxygen, the rest being nitrogen, so that, by ascertaining the diminution of the proportion in the flask, we can find how many cubic centimeters have been absorbed by the yeast. The author, however, has not given all the data necessary for accurate calculation.—D. C. R.〗 The weight of the yeast, in a state of dryness, was 0.035 gramme.
It follows that in the production of 35 milligrammes (0.524 grain) of yeast there was an absorption of 14 or 15 cc. (about 7/8 cub. in.) of oxygen, even supposing that the yeast was formed entirely under the influence of that gas: this is equivalent to not less than 414 cc. for 1 gramme of yeast (or about 33 cubic inches for every 20 grains).〖This number is probably too small; it is scarcely possible that the increase of weight in the yeast, even under the exceptional conditions of the experiment described, was not to some extent at least due to oxidation apart from free oxygen, inasmuch as some of the cells were covered by others. The increased weight of the yeast is always due to the action of two distinct modes of vital energy—activity, namely, in presence and activity in absence of air. We might endeavour to shorten the duration of the experiment still further, in which case we would still more assimilate the life of the yeast to that of ordinary moulds.〗
Such is the large volume of oxygen necessary for the development of one gramme of yeast when the plant can assimilate this gas after the manner of an ordinary fungus.
Let us now return to the first experiment described in the paragraph on page 278 in which a flask of three litres capacity was filled with fermentable liquid, which, when caused to ferment, yielded 2.25 grammes of yeast, under circumstances where it could not obtain a greater supply of free oxygen than 16.5 cc. (about one cubic inch). According to what we have just stated, if this 2.25 grammes (34 grains) of yeast had not been able to live without oxygen, in other words, if the original cells had been unable to multiply otherwise than by absorbing free oxygen, the amount of that gas required could not have been less than 2.25×414 cc., that is, 931.5 cc. (56.85 cubic inches). The greater part of the 2.25 grammes, therefore, had evidently been produced as the growth of an anaërobian plant.
Ordinary fungi likewise require large quantities of oxygen for their development, as we may readily prove by cultivating any mould in a closed vessel full of air, and then taking the weight of plant formed and measuring the volume of oxygen absorbed. To do this, we take a flask of the shape shown in FIG. 8, capable of holding about 300 cc. (10 1/2 fluid ounces), and containing a liquid adapted to the life of moulds. We boil this liquid, and seal the drawn-out point after the steam has expelled the air wholly or in part; we then open the flask in a garden or in a room. Should a fungus-spore enter the flask, as will invariably be the case in a certain number of flasks out of several used in the experiment, except under special circumstances, it will develop there and gradually absorb all the oxygen contained in the air of the flask. Measuring the volume of this air, and weighing, after drying, the amount of plant formed, we find that for a certain quantity of oxygen absorbed we have a certain weight of mycelium, or of mycelium together with its organs of fructification. In an experiment of this kind, in which the plant was weighed a year after its development, we found for 0.008 gramme (0.123 grain) of mycelium, dried at 100° C. (212° F.), an absorption that amounted to not less than 43 cc. (2.5 cubic inches) of oxygen at 25° C. These numbers, however, must vary sensibly with the nature of the mould employed, and also with the greater or less activity of its development, because the phenomena is complicated by the presence of accessory oxidations, such as we find in the case of mycoderma vini and aceti, to which cause the large absorption of oxygen in our last experiment may doubtless be attributed.〖In these experiments, in which the moulds remain for a long time in contact with a saccharine wort out of contact with oxygen—the oxygen being promptly absorbed by the vital action of the plant (see our Mémoire sur les Génerations dites Spontanées, p. 54, note)—there is no doubt that an appreciable quantity of alcohol is formed because the plant does not immediately lose its vital activity after the absorption of oxygen. A 300-cc. (10-oz.) flask, containing 100 cc. of must, after the air in it had been expelled by boiling, was opened and immediately re-closed on August 15th, 1873. A fungoid growth—a unique one, of greenish-grey colour—developed from spontaneous impregnation, and decolorized the liquid, which originally was of a yellowish-brown. Some large crystals, sparkling like diamonds, of neutral tartrate of lime, were precipitated. About a year afterwards, long after the death of the plant, we examined this liquid. It contained 0.3 gramme (4.6 grains) of alcohol, and 0.053 gramme (0.8 grain) of vegetable matter, dried at 100° C. (212° F.). We ascertained that the spores of the fungus were dead at the moment when the flask was opened. When sown, they did not develop in the least degree.〗
FIG. 8
The conclusions to be drawn from the whole of the preceding facts can scarcely admit of doubt. As for ourselves, we have no hesitation in finding them the foundation of the true theory of fermentation. In the experiments which we have described, fermentation by yeast, that is to say, by the type of ferments properly so called, is presented to us, in a word, as the direct consequence of the processes of nutrition, assimilation and life, when these are carried on without the agency of free oxygen. The heat required in the accomplishment of that work must necessarily have been borrowed from the decomposition of the fermentable matter, that is from the saccharine substance which, like other unstable substances, liberates heat in undergoing decomposition. Fermentation by means of yeast appears, therefore, to be essentially connected with the property possessed by this minute cellular plant of performing its respiratory functions, somehow or other, with oxygen existing combined in sugar. Its fermentative power—which power must not be confounded with the fermentative activity or the intensity of decomposition in a given time—varies considerably between two limits, fixed by the greatest and least possible access to free oxygen which the plant has in the process of nutrition. If we supply it with a sufficient quantity of free oxygen for the necessities of its life, nutrition, and respiratory combustions, in other words, if we cause it to live after the manner of a mould, properly so called, it ceases to be a ferment, that is, the ratio between the weight of the plant developed and that of the sugar decomposed, which forms its principal food, is similar in amount to that in the case of fungi.〖We find in M. Raulin's note that “the minimum ratio between the weight of sugar and the weight of organized matter, that is, the weight of fungoid growth which it helps to form, may be expressed as 10/3.2=3.1.” JULES RAULIN, Etudes chimiques sur la végétation. Recherches sur le développement d'une mucédinée dans un milieu artificiel, p. 192. Paris, 1870. We have seen in the case of yeast that this ratio may be as low as 4/1.〗 On the other hand, if we deprive the yeast of air entirely, or cause it to develop in a saccharine medium deprived of free oxygen, it will multiply just as if air were present, although with less activity, and under these circumstances its fermentative character will be most marked; under these circumstances, moreover, we shall find the greatest disproportion, all other conditions being the same, between the weight of yeast formed and the weight of sugar decomposed. Lastly, if free oxygen occurs in varying quantities, the ferment-power of the yeast may pass through all the degrees comprehended between the two extreme limits of which we have just spoken. It seems to us that we could not have a better proof of the direct relation that fermentation bears to life, carried on in the absence of free oxygen, or with a quantity of that gas insufficient for all the acts of nutrition and assimilation.
Another equally striking proof of the truth of this theory is the fact previously demonstrated that the ordinary moulds assume the character of a ferment when compelled to live without air, or with quantities of air too scant to permit of their organs having around them as much of that element as is necessary for their life as aërobian plants. Ferments, therefore, only possess in a higher degree a character which belongs to many common moulds, if not to all, and which they share, probably, more or less, with all living cells, namely the power of living either an aërobian or anaërobian life, according to the conditions under which they are placed.
It may be readily understood how, in their state of aërobian life, the alcoholic ferments have failed to attract attention. These ferments are only cultivated out of contact with air, at the bottom of liquids which soon become saturated with carbonic acid gas. Air is only present in the earlier developments of their germs, and without attracting the attention of the operator, whilst in their state of anaërobian growth their life and action are of prolonged duration. We must have recourse to special experimental apparatus to enable us to demonstrate the mode of life of alcoholic ferments under the influence of free oxygen; it is their state of existence apart from air, in the depths of liquids, that attracts all our attention. The results of their action are, however, marvellous, if we regard the products resulting from them, in the important industries of which they are the life and soul. In the case of ordinary moulds, the opposite holds good. What we want to use special experimental apparatus for with them, is to enable us to demonstrate the possibility of their continuing to live for a time out of contact with air, and all our attention, in their case, is attracted by the facility with which they develop under the influence of oxygen. Thus the decomposition of saccharine liquids, which is the consequence of the life of fungi without air, is scarcely perceptible, and so is of no practical importance. Their aërial life, on the other hand, in which they respire and accomplish their process of oxidation under the influence of free oxygen is a normal phenomenon, and one of prolonged duration which cannot fail to strike the least thoughtful of observers. We are convinced that a day will come when moulds will be utilised in certain industrial operations, on account of their power in destroying organic matter. The conversion of alcohol into vinegar in the process of acetification and the production of gallic acid by the action of fungi on wet gall nuts, are already connected with this kind of phenomena.〖We shall show, some day, that the processes of oxidation due to growth of fungi cause, in certain decompositions, liberation of ammonia to a considerable extent, and that by regulating their action we might cause them to extract the nitrogen from a host of organic débris, as also, by checking the production of such organisms, we might considerably increase the proportion of nitrates in the artificial nitrogenous substances. By cultivating the various moulds on the surface of damp bread in a current of air we have obtained an abundance of ammonia, derived from the decomposition of the albuminoids effected by the fungoid life. The decomposition of asparagus and several other animal or vegetable substances has given similar results.〗 On this last subject, the important work of M. Van Tieghem (Annales Scientifiques de l'Ecole Normale, vol. vi.) may be consulted.
The possibility of living without oxygen, in the case of ordinary moulds, is connected with certain morphological modifications which are more marked in proportion as this faculty is itself more developed. These changes in the vegetative forms are scarcely perceptible, in the case of penicillium and mycoderma vini, but they are very evident in the case of aspergillus, consisting of a marked tendency on the part of the submerged mycelial filaments to increase in diameter, and to develop cross partitions at short intervals, so that they sometimes bear a resemblance to chains of conidia. In mucor, again, they are very marked, the inflated filaments which, closely interwoven, present chains of cells, which fall off and bud, gradually producing a mass of cells. If we consider the matter carefully, we shall see that yeast presents the same characteristics.* * * *
It is a great presumption in favor of the truth of theoretical ideas when the results of experiments undertaken on the strength of those ideas are confirmed by various facts more recently added to science, and when those ideas force themselves more and more on our minds, in spite of a prima facie improbability. This is exactly the character of those ideas which we have just expounded. We pronounced them in 1861, and not only have they remained unshaken since, but they have served to foreshadow new facts, so that it is much easier to defend them in the present day than it was to do so fifteen years ago. We first called attention to them in various notes, which we read before the Chemical Society of Paris, notably at its meetings of April 12th and June 28th, 1861, and in papers in the Comptes rendus de l'Académie des Sciences. It may be of some interest to quote here, in its entirety, our communication of June 28th, 1861, entitled, “Influences of Oxygen on the Development of Yeast and on Alcoholic Fermentation,” which we extract from the Bulletin de la Société Chimique de Paris:—
“M. Pasteur gives the result of his researches on the fermentation of sugar and the development of yeast-cells, according as that fermentation takes place apart from the influence of free oxygen or in contact with that gas. His experiments, however, have nothing in common with those of Gay-Lussac, which were performed with the juice of grapes crushed under conditions where they would not be affected by air, and then brought into contact with oxygen.
“Yeast, when perfectly developed, is able to bud and grow in a saccharine and albuminous liquid, in the complete absence of oxygen or air. In this case but little yeast is formed, and a comparatively large quantity of sugar disappears—sixty or eighty parts for one of yeast formed. Under these conditions fermentation is very sluggish.
“If the experiment is made in contact with the air, and with a great surface of liquid, fermentation is rapid. For the same quantity of sugar decomposed much more yeast is formed. The air with which the liquid is in contact is absorbed by the yeast. The yeast develops very actively, but its fermentative character tends to disappear under these conditions; we find, that for one part of yeast formed, not more than from four to ten parts of sugar are transformed. The fermentative character of this yeast nevertheless continues, and produces even increased effects, if it is made to act on sugar apart from the influence of free oxygen.
“It seems, therefore, natural to admit that when yeast functions as a ferment by living apart from the influence of air, it derives oxygen from the sugar, and that this is the origin of its fermentative character.
“M. Pasteur explains the fact of the immense activity at the commencement of fermentations by the influence of the oxygen of the air held in solution in the liquids, at the time when the action commences. The author has found, moreover, that the yeast of beer sown in an albuminous liquid, such as yeast-water, still multiplies, even when there is not a trace of sugar in the liquid, provided always that atmospheric oxygen is present in large quantities. When deprived of air, under these conditions, yeast does not germinate at all. The same experiments may be repeated with albuminous liquid, mixed with a solution of non-fermentable sugar, such as ordinary crystallized milk-sugar. The results are precisely the same.
“Yeast formed thus in the absence of sugar does not change its nature; it is still capable of causing sugar to ferment, if brought to bear upon that substance apart from air. It must be remarked, however, that the development of yeast is effected with great difficulty when it has not a fermentable substance for its food. In short, the yeast of beer acts in exactly the same manner as an ordinary plant, and the analogy would be complete if ordinary plants had such an affinity for oxygen as permitted them to breathe by appropriating this element from unstable compounds, in which case, according to M. Pasteur, they would appear as ferments for those substances.
“M. Pasteur declares that he hopes to be able to realize this result, that is to say, to discover the conditions under which certain inferior plants may live apart from air in the presence of sugar, causing that substance to ferment as the yeast of beer would do.”
This summary and the preconceived views that it set forth have lost nothing of their exactness; on the contrary, time has strengthened them. The surmises of the last two paragraphs have received valuable confirmation from recent observations made by Messrs. Lechartier and Bellamy, as well as by ourselves, an account of which we must put before our readers. It is necessary, however, before touching upon this curious feature in connection with fermentations to insist on the accuracy of a passage in the preceding summary; the statement, namely, that yeast could multiply in an albuminous liquid, in which it found a non-fermentable sugar, milk-sugar, for example. The following is an experiment on this point:—On August 15th, 1875, we sowed a trace of yeast in 150 cc. (rather more than 5 fluid ounces) of yeast-water, containing 2 1/2 per cent. of milk-sugar. The solution was prepared in one of our double-necked flasks, with the necessary precautions to secure the absence of germs, and the yeast sown was itself perfectly pure. Three months afterwards, November 15th, 1875, we examined the liquid for alcohol; it contained only the smallest trace; as for the yeast (which had sensibly developed), collected and dried on a filter paper, it weighed 0.050 gramme (0.76 grain). In this case we have the yeast multiplying without giving rise to the least fermentation, like a fungoid growth, absorbing oxygen, and evolving carbonic acid, and there is no doubt that the cessation of its development in this experiment was due to the progressive deprivation of oxygen that occurred. As soon as the gaseous mixture in the flask consisted entirely of carbonic acid and nitrogen, the vitality of the yeast was dependent on, and in proportion to, the quantity of air which entered the flask in consequence of variations of temperature. The question now arose, was this yeast, which had developed wholly as an ordinary fungus, still capable of manifesting the character of a ferment? To settle this point we had taken the precaution on August 15th, 1875, of preparing another flask, exactly similar to the preceding one in every respect, and which gave results identical with those described. We decanted this November 15th, pouring some wort on the deposit of the plant, which remained in the flask. In less than five hours from the time we placed it in the oven, the plant started fermentation in the wort, as we could see by the bubbles of gas rising to form patches on the surface of the liquid. We may add that yeast in the medium which we have been discussing will not develop at all without air.
The importance of these results can escape no one; they prove clearly that the fermentative character is not an invariable phenomenon of yeast-life, they show that yeast is a plant which does not differ from ordinary plants, and which manifests its fermentative power solely in consequence of particular conditions under which it is compelled to live. It may carry on its life as a ferment or not, and after having lived without manifesting the slightest symptom of fermentative character, it is quite ready to manifest that character when brought under suitable conditions. The fermentative property, therefore, is not a power peculiar to cells of a special nature. It is not a permanent character of a particular structure, like, for instance, the property of acidity or alkalinity. It is a peculiarity dependent on external circumstances and on the nutritive conditions of the organism.