INTRODUCTORY NOTE
HERMANN LUDWIG FERDINAND VON HELMHOLTZ was born at Potsdam, near Berlin, on August 31, 1821. His father was a man of high culture, a teacher in the gymnasium, whose influence ensured to his son the foundations of a broad general education. His mother was a descendant from William Penn, the English Quaker.
Helmholtz early showed mathematical ability, and wished to devote his life to the study of physics; but practical considerations led him to take up medicine, and he became a surgeon in the Prussian army. He began the publication of original contributions to science in 1842, and for fifty-two years, till his death in 1894, he continued to produce in an unbroken stream. He held a succession of academic positions, teaching physiology at Königsberg, Bonn, and Heidelberg, and for the last twenty-three years of his life filling the chair of physics at Berlin.
The titles of his professorships, however, give a very inadequate idea of his range. His contributions to science cover medicine, physiology, optics, acoustics, mathematics, mechanics, and electricity. His interests in science and art came together in his work on esthetics, and he had a lively appreciation of painting, poetry, and music.
The practice of popular lecturing on scientific subjects was almost unknown in Germany when Helmholtz began, and he did much to give it dignity and to set a standard. His own lectures, as the reader of the following papers will perceive, are masterpieces of their kind. “The matter,” says a biographer, “is discussed by a master, who brings to bear upon it all his wealth of learning and research, while there is the ever-enduring interest that attaches to an exposition by one who is giving forth from his own treasury.” It is fortunate for the layman when a scientist and thinker of the first order has the skill and the inclination to share with the outside world the rich harvest of his brilliant and laborious research.
INTRODUCTION TO A SERIES OF LECTURES
DELIVERED AT CARLSRUHE IN THE
WINTER OF 1862-1863
AS I have undertaken to deliver here a series of lectures, I think the best way in which I can discharge that duty will be to bring before you, by means of a suitable example, some view of the special character of those sciences to the study of which I have devoted myself. The natural sciences, partly in consequence of their practical applications, and partly from their intellectual influence on the last four centuries, have so profoundly, and with such increasing rapidity, transformed all the relations of the life of civilised nations; they have given these nations such increase of riches, of enjoyment of life, of the preservation of health, of means of industrial and of social intercourse, and even such increase of political power, that every educated man who tries to understand the forces at work in the world in which he is living, even if he does not wish to enter upon the study of a special science, must have some interest in that peculiar kind of mental labour, which works and acts in the sciences in question.
On a former occasion I have already discussed the characteristic differences which exist between the natural and the mental sciences as regards the kind of scientific work. I then endeavoured to show that it is more especially in the thorough conformity with law which natural phenomena and natural products exhibit, and in the comparative ease with which laws can be stated, that this difference exists. Not that I wish by any means to deny, that the mental life of individuals and peoples is also in conformity with law, as is the object of philosophical, philological, historical, moral, and social sciences to establish. But in mental life, the influences are so interwoven, that any definite sequence can but seldom be demonstrated. In Nature the converse is the case. It has been possible to discover the law of the origin and progress of many enormously extended series of natural phenomena with such accuracy and completeness that we can predict their future occurrence with the greatest certainty; or in cases in which we have power over the conditions under which they occur, we can direct them just according to our will. The greatest of all instances of what the human mind can effect by means of a well-recognised law of natural phenomena is that afforded by modern astronomy. The one simple law of gravitation regulates the motions of the heavenly bodies not only of our own planetary system, but also of the far more distant double stars; from which, even the ray of light, the quickest of all messengers, needs years to reach our eye; and, just on account of this simple conformity with law, the motions of the bodies in question can be accurately predicted and determined both for the past and for future years and centuries to a fraction of a minute.
On this exact conformity with law depends also the certainty with which we know how to tame the impetuous force of steam, and to make it the obedient servant of our wants. On this conformity depends, moreover, the intellectual fascination which chains the physicist to his subjects. It is an interest of quite a different kind to that which mental and moral sciences afford. In the latter it is man in the various phases of his intellectual activity who chains us. Every great deed of which history tells us, every mighty passion which art can represent, every picture of manners, of civic arrangements, of the culture of peoples of distant lands or of remote times, seizes and interests us, even if there is no exact scientific connection among them. We continually find points of contact and comparison in our conceptions and feelings; we get to know the hidden capacities and desires of the mind, which in the ordinary peaceful course of civilised life remain unawakened.
It is not to be denied that, in the natural sciences, this kind of interest is wanting. Each individual fact, taken by itself, can indeed arouse our curiosity or our astonishment, or be useful to us in its practical applications. But intellectual satisfaction we obtain only from a connection of the whole, just from its conformity with law. Reason we call that faculty innate in us of discovering laws and applying them with thought. For the unfolding of the peculiar forces of pure reason in their entire certainty and in their entire bearing, there is no more suitable arena than inquiry into Nature in the wider sense, the mathematics included. And it is not only the pleasure at the successful activity of one of our most essential mental powers; and the victorious subjections to the power of our thought and will of an external world, partly unfamiliar, and partly hostile, which is the reward of this labour; but there is a kind, I might almost say, of artistic satisfaction, when we are able to survey the enormous wealth of Nature as a regularly-ordered whole—a kosmos, an image of the logical thought of our own mind.
The last decades of scientific development have led us to the recognition of a new universal law of all natural phenomena, which, from its extraordinarily extended range, and from the connection which it constitutes between natural phenomena of all kinds, even of the remotest times and the most distant places, is especially fitted to give us an idea of what I have described as the character of the natural sciences, which I have chosen as the subject of this lecture.
This law is the Law of the Conservation of Force, a term the meaning of which I must first explain. It is not absolutely new; for individual domains of natural phenomena it was enunciated by Newton and Daniel Bernoulli; and Rumford and Humphry Davy have recognised distinct features of its presence in the laws of heat.
The possibility that it was of universal application was first stated by Dr. Julius Robert Mayer, a Schwabian physician (now living in Heilbronn), in the year 1842, while almost simultaneously with, and independently of him, James Prescot Joule, an English manufacturer, made a series of important and difficult experiments on the relation of heat to mechanical force, which supplied the chief points in which the comparison of the new theory with experience was still wanting.
The law in question asserts, that the quantity of force which can be brought into action in the whole of Nature is unchangeable, and can neither be increased nor diminished. My first object will be to explain to you what is understood by quantity of force; or, as the same idea is more popularly expressed with reference to its technical application, what we call amount of work in the mechanical sense of the word.
The idea of work for machines, or natural processes, is taken from comparison with the working power of man; and we can therefore best illustrate from human labour the most important features of the question with which we are concerned. In speaking of the work of machines and of natural forces we must, of course, in this comparison eliminate anything in which activity of intelligence comes into play. The latter is also capable of the hard and intense work of thinking, which tries a man just as muscular exertion does. But whatever of the actions of intelligence is met with in the work of machines, of course is due to the mind of the constructor and cannot be assigned to the instrument at work.
Now, the external work of man is of the most varied kind as regards the force or ease, the form and rapidity, of the motions used on it, and the kind of work produced. But both the arm of the blacksmith who delivers his powerful blows with the heavy hammer, and that of the violinist who produces the most delicate variations in sound, and the hand of the lace-maker who works with threads so fine that they are on the verge of the invisible, all these acquire the force which moves them in the same manner and by the same organs, namely, the muscles of the arms. An arm the muscles of which are lamed is incapable of doing any work; the moving force of the muscle must be at work in it, and these must obey the nerves, which bring to them orders from the brain. That member is then capable of the greatest variety of motions; it can compel the most varied instruments to execute the most diverse tasks.
Just so it is with machines: they are used for the most diversified arrangements. We produce by their agency an infinite variety of movements, with the most various degrees of force and rapidity, from powerful steam hammers and rolling mills, where gigantic masses of iron are cut and shaped like butter, to spinning and weaving frames, the work of which rivals that of the spider. Modern mechanism has the richest choice of means of transferring the motion of one set of rolling wheels to another with greater or less velocity; of changing the rotating motion of wheels into the up-and-down motion of the piston rod, of the shuttle, of falling hammers and stamps; or, conversely, of changing the latter into the former; or it can, on the other hand, change movements of uniform into those of varying velocity, and so forth. Hence this extraordinarily rich utility of machines for so extremely varied branches of industry. But one thing is common to all these differences; they all need a moving force, which sets and keeps them in motion, just as the works of the human hand all need the moving force of the muscles.
Now, the work of the smith requires a far greater and more intense exertion of the muscles than that of the violin player; and there are in machines corresponding differences in the power and duration of the moving force required. These differences, which correspond to the different degree of exertion of the muscles in human labour, are alone what we have to think of when we speak of the amount of work of a machine. We have nothing to do here with the manifold character of the actions and arrangements which the machines produce; we are only concerned with an expenditure of force.
This very expression which we use so fluently, ‘expenditure of force,’ which indicates that the force applied has been expended and lost, leads us to a further characteristic analogy between the effects of the human arm and those of machines. The greater the exertion, and the longer it lasts, the more is the arm tired, and the more is the store of its moving force for the time exhausted. We shall see that this peculiarity of becoming exhausted by work is also met with in the moving forces of inorganic nature; indeed, that this capacity of the human arm of being tired is only one of the consequences of the law with which we are now concerned. When fatigue sets in, recovery is needed, and this can only be effected by rest and nourishment. We shall find that also in the inorganic moving forces, when their capacity for work is spent, there is a possibility of reproduction, although in general other means must be used to this end than in the case of the human arm.
From the feeling of exertion and fatigue in our muscles, we can form a general idea of what we understand by amount of work; but we must endeavour, instead of the indefinite estimate afforded by this comparison, to form a clear and precise idea of the standard by which we have to measure the amount of work. This we can do better by the simplest inorganic moving forces than by the actions of our muscles, which are a very complicated apparatus, acting in an extremely intricate manner.
Let us now consider that moving force which we know best, and which is simplest—gravity. It acts, for example, as such in those clocks which are driven by a weight. This weight, fastened to a string, which is wound round a pulley connected with the first toothed wheel of the clock, cannot obey the pull of gravity without setting the whole clockwork in motion. Now I must beg you to pay special attention to the following points: the weight cannot put the clock in motion without itself sinking; did the weight not move, it could not move the clock, and its motion can only be such a one as obeys the action of gravity. Hence, if the clock is to go, the weight must continually sink lower and lower, and must at length sink so far that the string which supports it is run out. The clock then stops. The usual effect of its weight is for the present exhausted. Its gravity is not lost or diminished; it is attracted by the earth as before, but the capacity of this gravity to produce the motion of the clockwork is lost. It can only keep the weight at rest in the lowest point of its path, it cannot farther put it in motion.
But we can wind up the clock by the power of the arm, by which the weight is again raised. When this has been done, it has regained its former capacity, and can again set the clock in motion.
We learn from this that a raised weight possesses a moving force, but that it must necessarily sink if this force is to act; that by sinking, this moving force is exhausted, but by using another extraneous moving force—that of the arm—its activity can be restored.
The work which the weight has to perform in driving the clock is not indeed great. It has continually to overcome the small resistances which the friction of the axles and teeth, as well as the resistance of the air, oppose to the motion of the wheels, and it has to furnish the force for the small impulses and sounds which the pendulum produces at each oscillation. If the weight is detached from the clock, the pendulum swings for a while before coming to a rest, but its motion becomes each moment feebler, and ultimately ceases entirely, being gradually used up by the small hindrances I have mentioned. Hence, to keep the clock going, there must be a moving force, which, though small, must be continually at work. Such a one is the weight.
We get, moreover, from this example, a measure for the amount of work. Let us assume that a clock is driven by a weight of a pound, which falls five feet in twenty-four hours. If we fix ten such clocks, each with a weight of one pound, then ten clocks will be driven twenty-four hours; hence, as each has to overcome the same resistances in the same time as the others, ten times as much work is performed for ten pounds fall through five feet. Hence, we conclude that the height of the fall being the same, the work increases directly as the weight.
Now, if we increase the length of the string so that the weight runs down ten feet, the clock will go two days instead of one; and, with double the height of fall, the weight will overcome on the second day the same resistances as on the first, and will therefore do twice as much work as when it can only run down five feet. The weight being the same, the work increases as the height of fall. Hence, we may take the product of the weight into the height off all as a measure of work, at any rate, in the present case. The application of this measure is, in fact, not limited to the individual case, but the universal standard adopted in manufactures for measuring magnitude or work is a foot pound—that is, the amount of work which a pound raised through a foot can produce.〖This is the technical measure of work; to convert it into scientific measure it must be multiplied by the intensity of gravity.〗
We may apply this measure of work to all kinds of machines, for we should be able to set them all in motion by means of a weight sufficient to turn a pulley. We could thus always express the magnitude of any driving force, for any given machine, by the magnitude and height of fall of such a weight as would be necessary to keep the machine going with its arrangements until it had performed a certain work, Hence it is that the measurement of work by foot pounds is universally applicable. The use of such a weight as a driving force would not indeed be practically advantageous in those cases in which we were compelled to raise it by the power of our own arm; it would in that case be simpler to work the machine by the direct action of the arm. In the clock we use a weight so that we need not stand the whole day at the clockwork, as we should have to do to move it directly. By winding up the clock we accumulate a store of working capacity in it, which is sufficient for the expenditure of the next twenty-four hours.
The case is somewhat different when Nature herself raises the weight, which then works for us. She does not do this with solid bodies, at least not with such regularity as to be utilised; but she does it abundantly with water, which, being raised to the tops of mountains by meteorological processes, returns in streams from them. The gravity of water we use as moving force, the most direct application being in what are called overshot wheels, one of which is represented in FIG. 90. Along the circumference of such a wheel are a series of buckets, which act as receptacles for the water, and, on the side turned to the observer, have the tops uppermost; on the opposite side the tops of the buckets are upside-down. The water flows at M into the buckets of the front of the wheel, and at F, where the mouth begins to incline downwards, it flows out. The buckets on the circumference are filled on the side turned to the observer, and empty on the other side. Thus the former are weighted by the water contained in them, the latter not; the weight of the water acts continuously on only one side of the wheel, draws this down, and thereby turns the wheel; the other side of the wheel offers no resistance, for it contains no water. It is thus the weight of the falling water which turns the wheel, and furnishes the motive power. But you will at once see that the mass of water which turns the wheel must necessarily fall in order to do so, and that though, when it has reached the bottom, it has lost none of its gravity, it is no longer in a position to drive the wheel, if it is not restored to its original position, either by the power of the human arm or by means of some other natural force. If it can flow from the mill-stream to still lower levels, it may be used to work other wheels. But when it has reached its lowest level, the sea, the last remainder of the moving force is used up, which is due to gravity—that is, to the attraction of the earth, and it cannot act by its weight until it has been again raised to a high level. As this is actually effected by meteorological processes, you will at once observe that these are to be considered as sources of moving force.
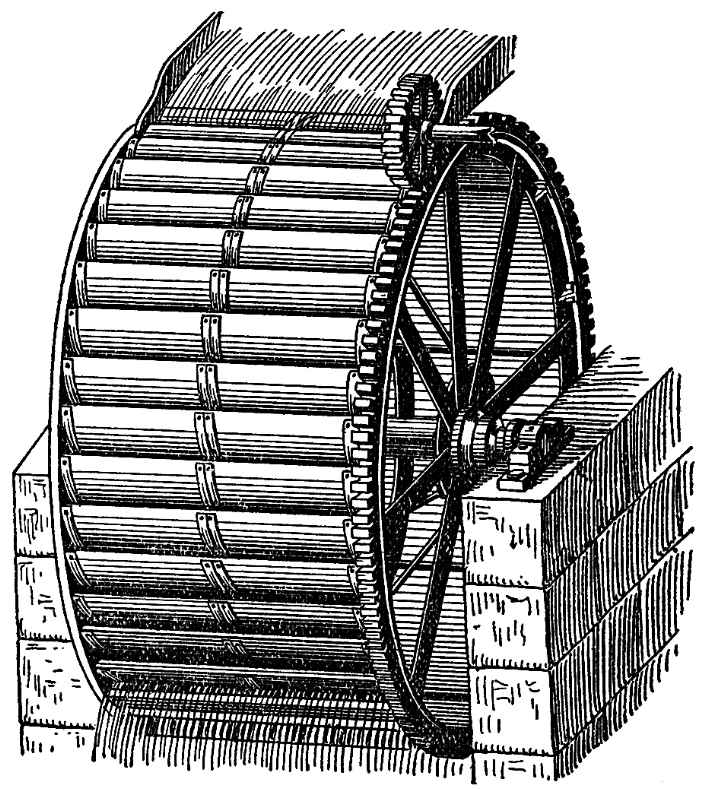
FIG. 90
Water-power was the first inorganic force which man learnt to use instead of his own labour or of that of domestic animals. According to Strabo, it was known to King Mithridates of Pontus, who was also otherwise celebrated for his knowledge of Nature; near his palace there was a water wheel. Its use was first introduced among the Romans in the time of the first Emperors. Even now we find water mills in all mountains, valleys, or wherever there are rapidly-flowing regularly-filled brooks and streams. We find water power used for all purposes which can possibly be effected by machines. It drives mills which grind corn, sawmills, hammers, and oil presses, spinning frames and looms, and so forth. It is the cheapest of all motive powers, it flows spontaneously from the inexhaustible stores of Nature; but it is restricted to a particular place, and only in mountainous countries is it present in any quantity; in level countries extensive reservoirs are necessary for damming the rivers to produce any amount of water-power.
Before passing to the discussion of other motive forces I must answer an objection which may readily suggest itself. We all know that there are numerous machines, systems of pulleys, levers and cranes, by the aid of which heavy burdens may be lifted by a comparatively small expenditure of force. We have all of us often seen one or two workmen hoist heavy masses of stones to great heights, which they would be quite unable to do directly; in like manner, one or two men, by means of a crane, can transfer the largest and heaviest chests from a ship to the quay. Now, it may be asked, If a large, heavy weight had been used for driving a machine, would it not be very easy, by means of a crane or a system of pulleys, to raise it anew, so that it could again be used as a motor, and thus acquire motive power, without being compelled to use a corresponding exertion in raising the weight?
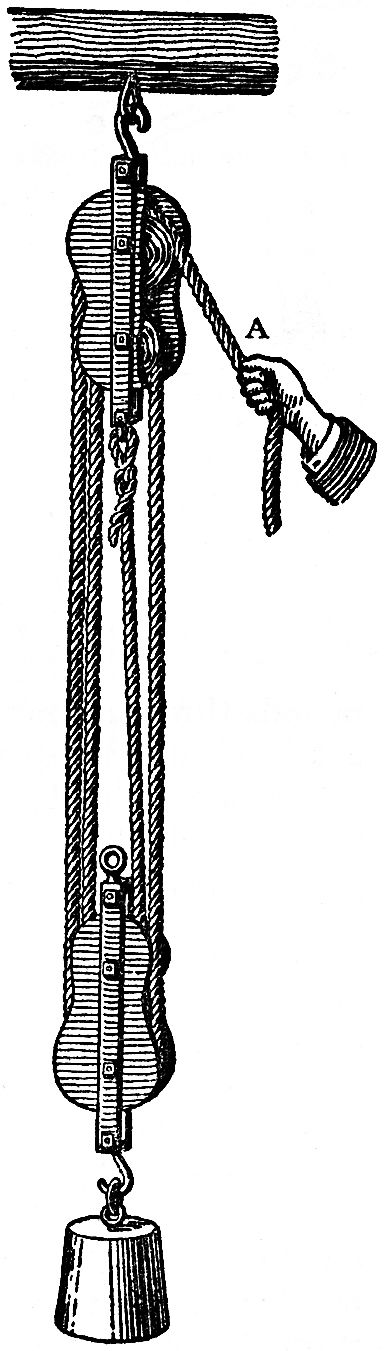
FIG. 91
The answer to this is, that all these machines, in that degree in which for the moment they facilitate the exertion, also prolong it, so that by their help no motive power is ultimately gained. Let us assume that four labourers have to raise a load of four hundredweight by means of a rope passing over a single pulley. Every time the rope is pulled down through four feet, the load is also raised through four feet. But now, for the sake of comparison, let us suppose the same load hung to a block of four pulleys, as represented in FIG. 91. A single labourer would not be able to raise the load by the same exertion of force as each one of the four put forth. But when he pulls the rope through four feet, the load only rises one foot, for the length through which he pulls the rope, at a, is uniformly distributed in the block over four ropes, so that each of these is only shortened by a foot. To raise the load, therefore, to the same height, the one man must necessarily work four times as long as the four together did. But the total expenditure of work is the same, whether four labourers work for a quarter of an hour or one works for an hour.
If, instead of human labour, we introduce the work of a weight, and hang to the block a load of 400, and at a, where otherwise the labourer works, a weight of 100 pounds, the block is then in equilibrium, and, without any appreciable exertion of the arm, may be set in motion. The weight of 100 pounds sinks, that of 400 rises. Without any measurable expenditure of force, the heavy weight has been raised by the sinking of the smaller one. But observe that the smaller weight will have sunk through four times the distance that the greater one has risen. But a fall of 100 pounds through four feet is just as much 400 foot-pounds as a fall of 400 pounds through one foot.
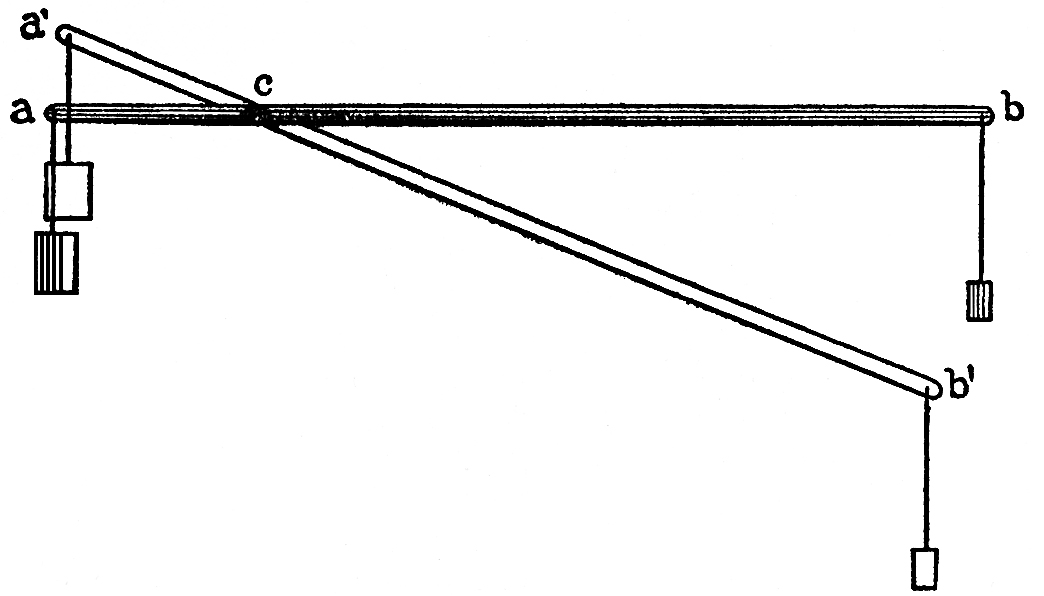
FIG. 92
The action of levers in all their various modifications is precisely similar. Let a b, FIG. 92, be a simple lever, supported at c, the arm c b being four times as long as the other arm a c. Let a weight of one pound be hung at b, and a weight of four pounds at a, the lever is then in equilibrium, and the least pressure of the finger is sufficient, without any appreciable exertion of force, to place it in the position a(1)b(1), in which the heavy weight of four pounds has been raised, while the one-pound weight has sunk. But here, also, you will observe no work has been gained, for while the heavy weight has been raised through one inch, the lighter one has fallen through four inches; and four pounds through one inch is, as work, equivalent to the product of one pound through four inches.

H. L. F. Helmholtz
Most other fixed parts of machines may be regarded as modified and compound levers; a toothed-wheel, for instance as a series of levers, the ends of which are represented by the individual teeth, and one after the other of which is put in activity in the degree in which the tooth in question seizes or is seized by the adjacent pinion. Take, for instance, the crabwinch, represented in FIG. 93. Suppose the pinion on the axis of the barrel of the winch has twelve teeth, and the toothed-wheel, H H, seventy-two teeth, that is, six times as many as the former. The winch must now be turned round six times before the toothed-wheel, H, and the barrel, D, have made one turn, and before the rope which raises the load has been lifted by a length equal to the circumference of the barrel. The workman thus requires six times the time, though to be sure only one-sixth of the exertion, which he would have to use if the handle were directly applied to the barrel, D. In all these machines, and parts of machines, we find it confirmed that in proportion as the velocity of the motion increases its power diminishes, and that when the power increases the velocity diminishes, but that the amount of work is never thereby increased.
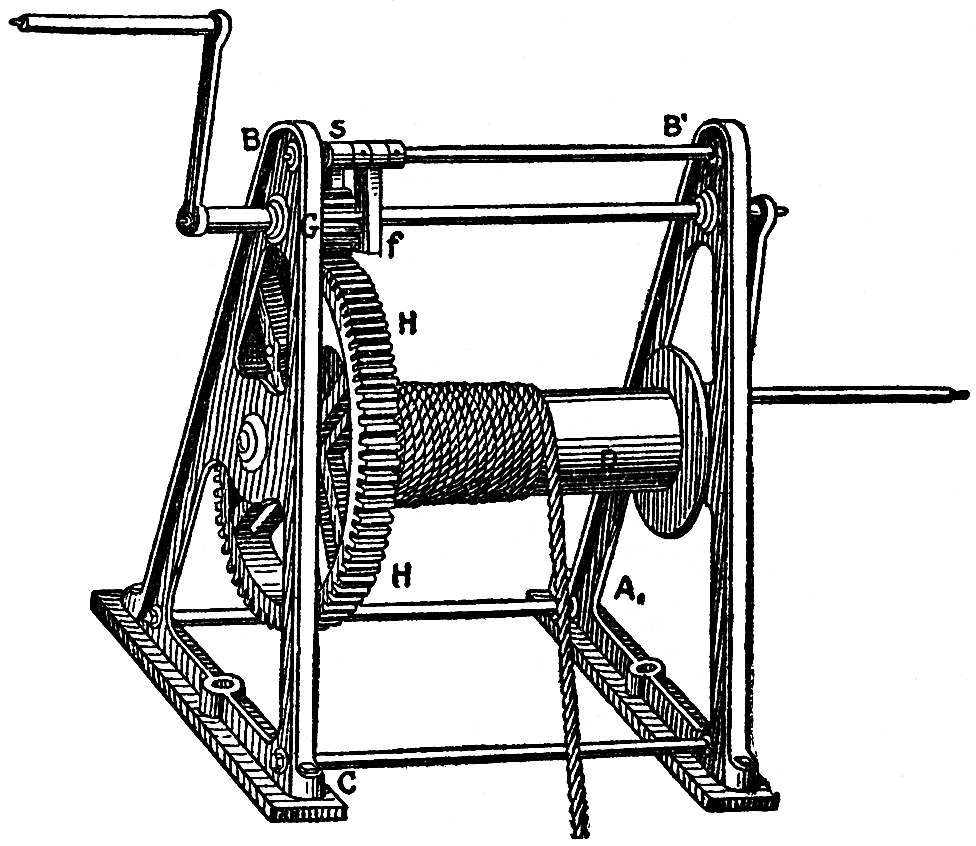
FIG. 93
In the overshot mill-wheel, described above, water acts by its weight. But there is another form of mill wheels, what is called the undershot wheel, in which it only acts by its impact, as represented in FIG. 94. These are used where the height from which the water comes is not great enough to flow on the upper part of the wheel. The lower part of undershot wheels dips in the flowing water which strikes against their float-boards and carries them along. Such wheels are used in swift-flowing streams which have a scarcely perceptible fall, as, for instance, on the Rhine. In the immediate neighborhood of such a wheel, the water need not necessarily have a great fall if it only strikes with considerable velocity. It is the velocity of the water, exerting an impact against the float-boards, which acts in this case, and which produces the motive power.
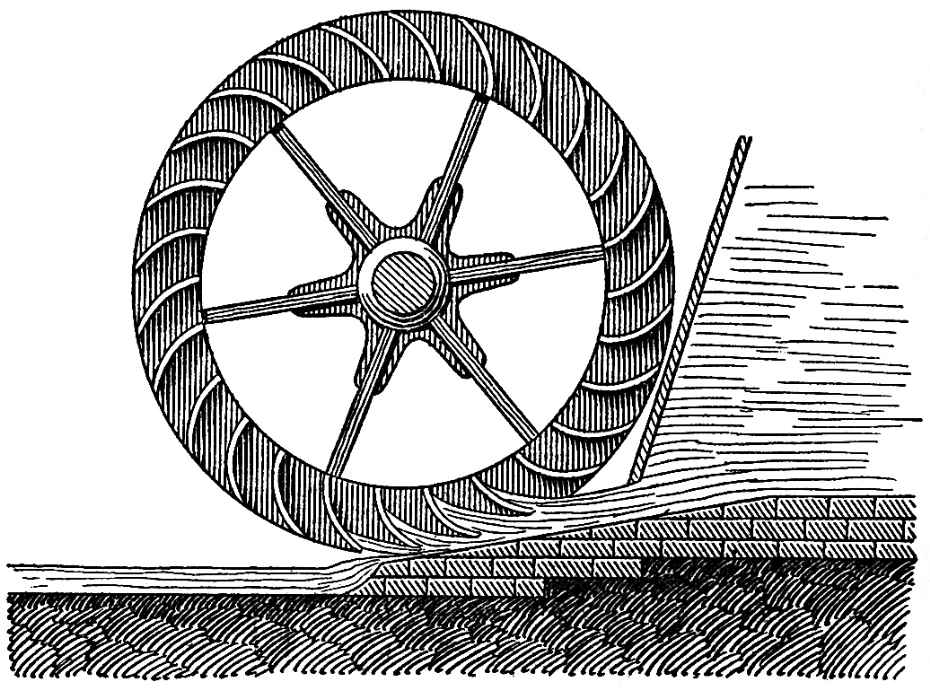
FIG. 94
Windmills, which are used in the great plains of Holland and North Germany to supply the want of falling water, afford another instance of the action of velocity. The sails are driven by air in motion—by wind. Air at rest could just as little drive a windmill as water at rest a water-wheel. The driving force depends here on the velocity of moving masses.
A bullet resting in the hand is the most harmless thing in the world; by its gravity it can exert no great effect; but when fired and endowed with great velocity it drives through all obstacles with the most tremendous force.
If I lay the head of a hammer gently on a nail, neither its small weight nor the pressure of my arm is quite sufficient to drive the nail into the wood; but if I swing the hammer and allow it to fall with great velocity, it acquires a new force, which can overcome far greater hindrances.
These examples teach us that the velocity of a moving mass can act as motive force. In mechanics, velocity in so far as it is motive force, and can produce work, is called vis viva. The name is not well chosen; it is too apt to suggest to us the force of living beings. Also in this case you will see, from the instances of the hammer and of the bullet, that velocity is lost, as such, when it produces working power. In the case of the water-mill, or of the windmill, a more careful investigation of the moving masses of water and air is necessary to prove that part of their velocity has been lost by the work which they have performed.
The relation of velocity to working power is most simply and clearly seen in a simple pendulum, such as can be constructed by any weight which we suspend to a cord. Let M, FIG. 95, be such a weight, of a spherical form: A B, a horizontal line drawn through the centre of the sphere; P the point at which the cord is fastened. If now I draw the weight M on one side towards A, it moves in the arc M a, the end of which, a, is somewhat higher than the point A in the horizontal line. The weight is thereby raised to the height A a. Hence my arm must exert a certain force to bring the weight to a. Gravity resists this motion, and endeavours to bring back the weight to M, the lowest point which it can reach.
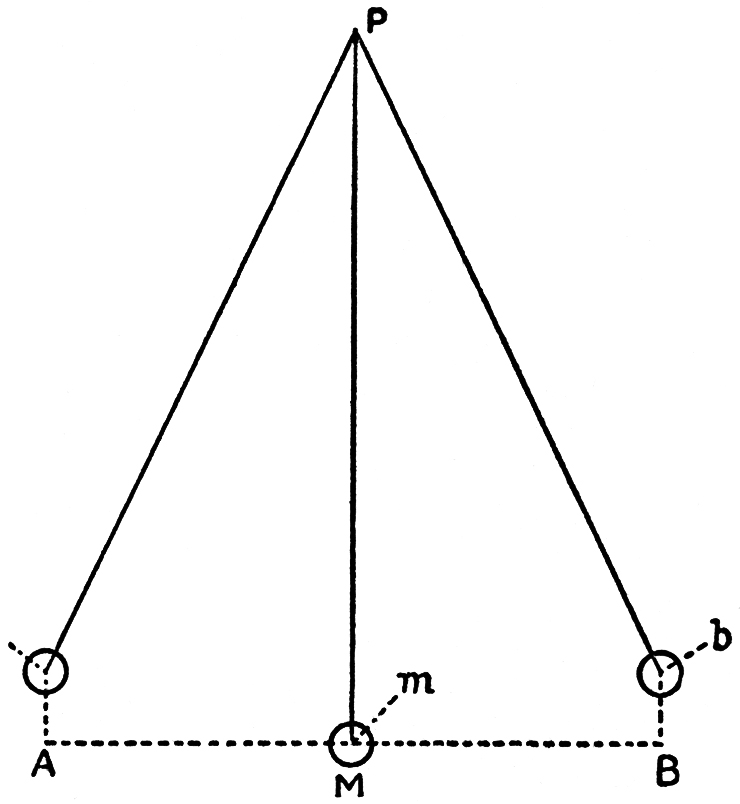
FIG. 95
Now, if after I have brought the weight to a I let it go, it obeys this force of gravity and returns to M, arrives there with a certain velocity, and no longer remains quietly hanging at M as it did before, but swings beyond M towards b, where its motion stops as soon as it has traversed on the side of B an arc equal in length to that on the side of A, and after it has risen to a distance B b above the horizontal line, which is equal to the height A a, to which my arm had previously raised it. In b the pendulum returns, swings the same way back through M towards a, and so on, until its oscillations are gradually diminished, and ultimately annulled by the resistance of the air and by friction.
You see here that the reason why the weight, when it comes from a to M, and does not stop there, but ascends to b, in opposition to the action of gravity, is only to be sought in its velocity. The velocity which it has acquired in moving from the height A a is capable of again raising it to an equal height, B b. The velocity of the moving mass, M, is thus capable of raising this mass; that is to say, in the language of mechanics, of performing work. This would also be the case if we had imparted such a velocity to the suspended weight by a blow.
From this we learn further how to measure the working power of velocity—or, what is the same thing, the vis viva of the moving mass. It is equal to the work, expressed in foot-pounds, which the same mass can exert after its velocity has been used to raise it, under the most favourable circumstances, to as great a height as possible.〖The measure of vis viva in theoretical mechanics is half the product of the weight into the square of the velocity. To reduce it to the technical measure of the work we must divide it by the intensity of gravity; that is, by the velocity at the end of the first second of a freely falling body.〗 This does not depend on the direction of the velocity; for if we swing a weight attached to a thread in a circle, we can even change a downward motion into an upward one.
The motion of the pendulum shows us very distinctly how the forms of working power hitherto considered—that of a raised weight and that of a moving mass—may merge into one another. In the points a and b, FIG. 95, the mass has no velocity; at the point M it has fallen as far as possible, but possesses velocity. As the weight goes from a to m the work of the raised weight is changed into vis viva; as the weight goes further m to b the vis viva is changed into the work of a raised weight. Thus the work which the arm originally imparted to the pendulum is not lost in these oscillations provided we may leave out of consideration the influence of the resistance of the air and of friction. Neither does it increase, but it continually changes the form of its manifestation.
Let us now pass to other mechanical forces, those of elastic bodies. Instead of the weights which drive our clocks, we find in timepieces and in watches, steel springs which are coiled in winding up the clock, and are uncoiled by the working of the clock. To coil up the spring we consume the force of the arm; this has to overcome the resisting elastic force of the spring as we wind it up, just as in the clock we have to overcome the force of gravity which the weight exerts. The coiled spring can, however, perform work; it gradually expends this acquired capability in driving the clock-work.
If I stretch a crossbow and afterwards let it go, the stretched string moves the arrow; it imparts to it force in the form of velocity. To stretch the cord my arm must work for a few seconds; this work is imparted to the arrow at the moment it is shot off. Thus the cross-bow concentrates into an extremely short time the entire work which the arm had communicated in the operation of stretching; the clock, on the contrary, spreads it over one or several days. In both cases no work is produced which my arm did not originally impart to the instrument; it is only expended more conveniently.
The case is somewhat different if by any other natural process I can place an elastic body in a state of tension without having to exert my arm. This is possible and is most easily observed in the case of gases.
If, for instance, I discharge a firearm loaded with gunpowder, the greater part of the mass of the powder is converted into gases at a very high temperature, which have a powerful tendency to expand, and can only be retained in the narrow space in which they are formed, by the exercise of the most powerful pressure. In expanding with enormous force they propel the bullet, and impart to it a great velocity, which we have already seen is a form of work.
In this case, then, I have gained work which my arm has not performed. Something, however, has been lost—the gunpowder, that is to say, whose constituents have changed into other chemical compounds, from which they cannot, without further ado, be restored to their original condition. Here, then, a chemical change has taken place, under the influence of which work has been gained.
Elastic forces are produced in gases by the aid of heat, on a far greater scale.
Let us take, as the most simple instance, atmospheric air. In FIG. 96 an apparatus is represented such as Regnault used for measuring the expansive force of heated gases. If not great accuracy is required in the measurement, the apparatus may be arranged more simply. At C is a glass globe filled with dry air, which is placed in a metal vessel, in which it can be heated by steam. It is connected with the U-shaped tube, S s, which contains a liquid, and the limbs of which communicate with each other when the stopcock R is closed. If the liquid is in equilibrium in the tube S s when the globe is cold, it rises in the leg s, and ultimately overflows when the globe is heated. If, on the contrary, when the globe is heated, equilibrium be restored by allowing some of the liquid to flow out at R, as the globe cools it will be drawn up towards n. In both cases liquid is raised, and work thereby produced.
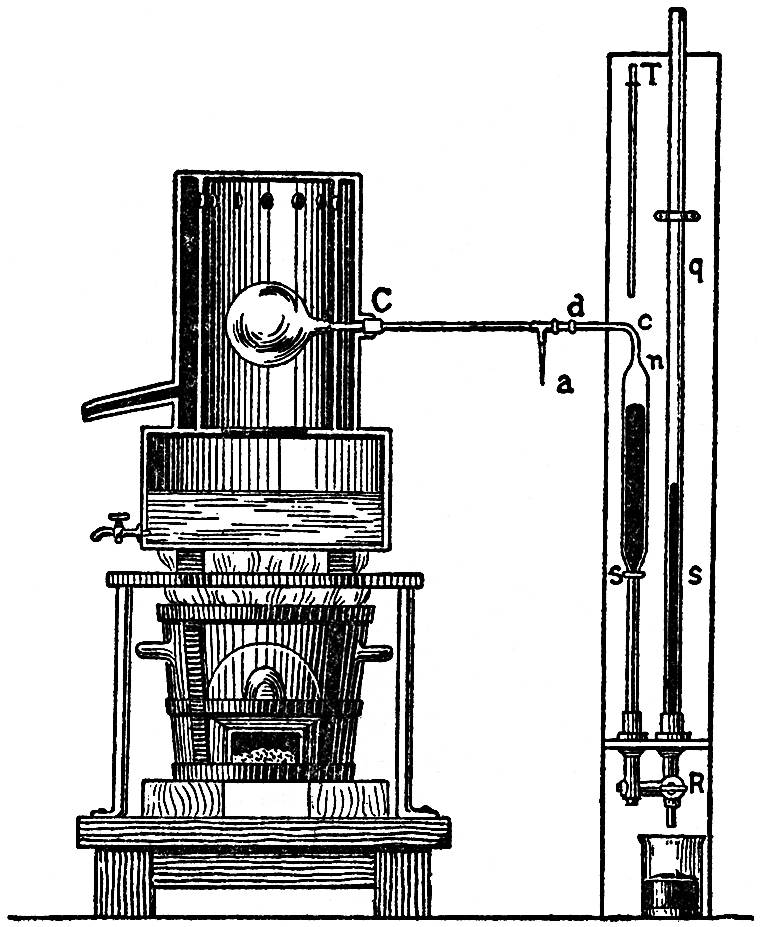
FIG. 96
The same experiment is continuously repeated on the largest scale in steam engines, though, in order to keep up a continual disengagement of compressed gases from the boiler, the air in the globe in FIG. 96, which would soon reach the maximum of its expansion, is replaced by water, which is gradually changed into steam by the application of heat. But steam, so long as it remains as such, is an elastic gas which endeavours to expand exactly like atmospheric air. And instead of the column of liquid which was raised in our last experiment, the machine is caused to drive a solid piston which imparts its motion to other parts of the machine. Fig. 97 represents a front view of the working parts of the high-pressure engine, and Fig. 98 a section. The boiler in which steam is generated is not represented; the steam passes through the tube z z, FIG. 98, to the cylinder A A, in which moves a tightly fitting piston C. The parts between the tube z z and the cylinder A A, that is the slide valve in the valve-chest K K, and the two tubes d and e allow the steam to pass first below and then above the piston, while at the same time the steam has free exit from the other half of the cylinder. When the steam passes under the piston, it forces it upward; when the piston has reached the top of its course the position of the valve in K K changes, and the steam passes above the piston and forces it down again. The piston-rod acts by means of the connecting-rod P, on the crank Q of the flywheel X and sets this in motion. By means of the rod s, the motion of the rod regulates the opening and closing of the valve. But we need not here enter into those mechanical arrangements, however ingeniously they have been devised. We are only interested in the manner in which heat produces elastic vapour, and how this vapour, in its endeavor to expand, is compelled to move the solid parts of the machine, and furnish work.
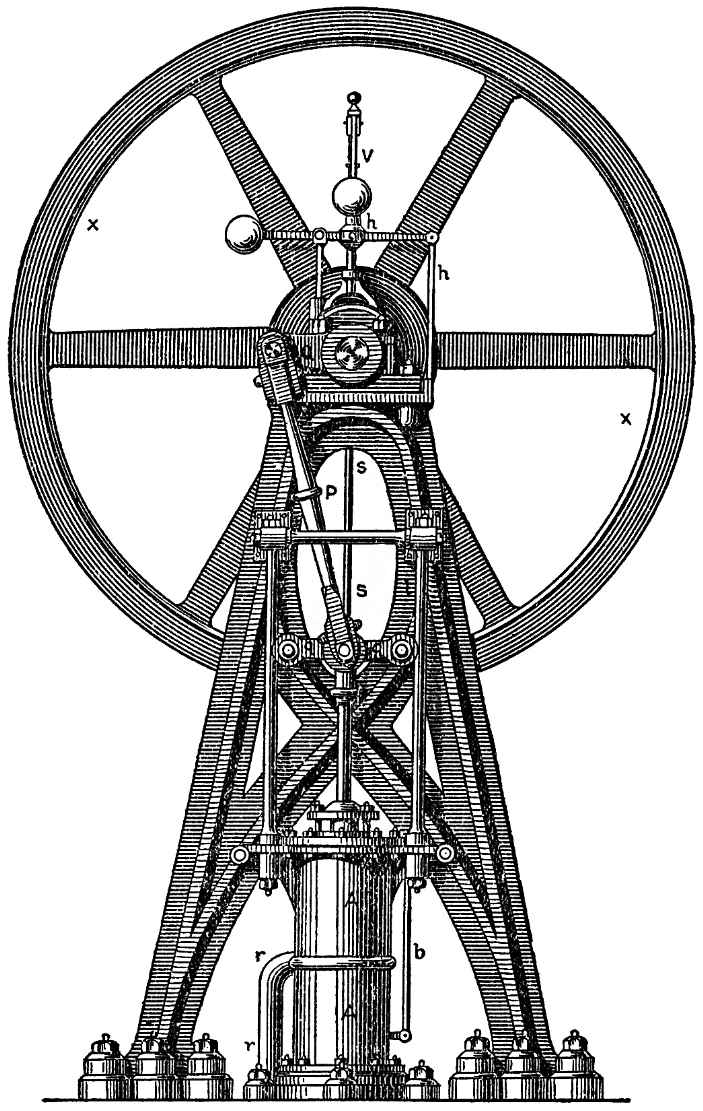
FIG. 97
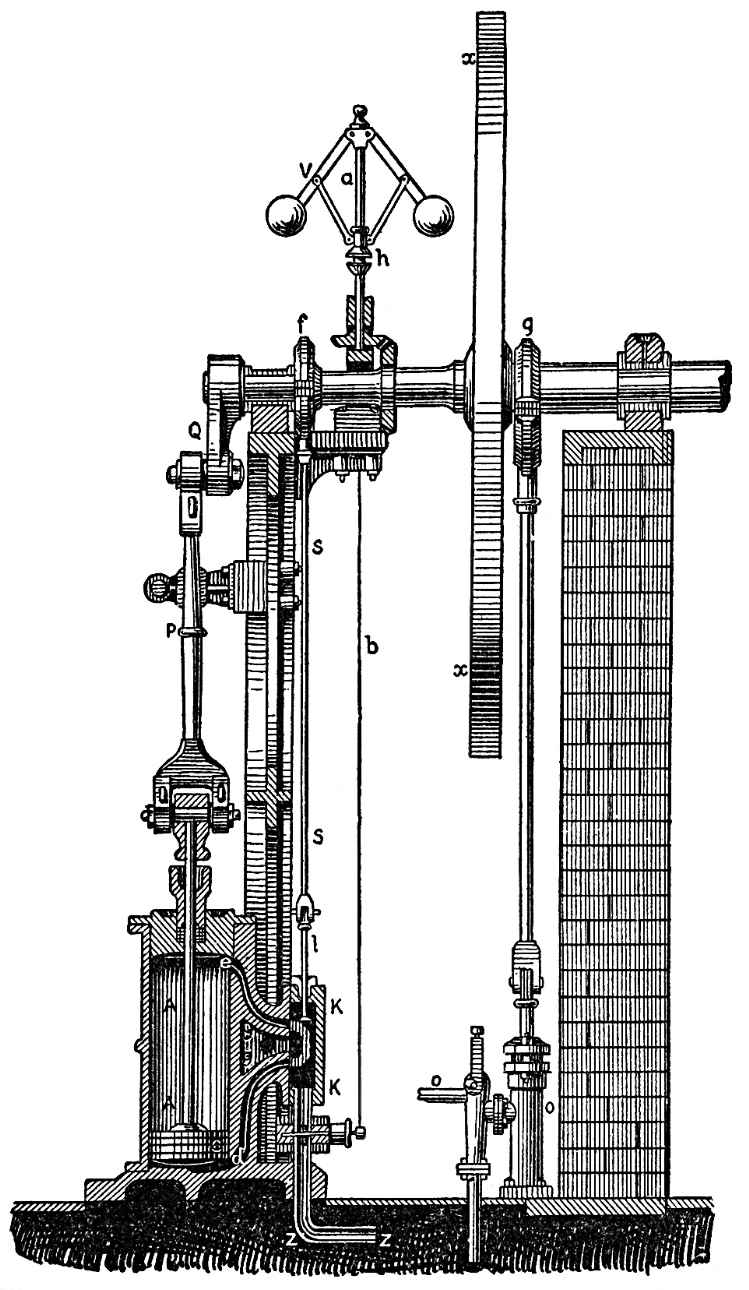
FIG. 98
You all know how powerful and varied are the effects of which steam-engines are capable; with them has really begun the great development of industry which has characterised our century before all others. Its most essential superiority over motive powers formerly known is that it is not restricted to a particular place. The store of coal and the small quantity of water which are the sources of its power can be brought everywhere, and steam engines can even be made movable, as is the case with steam ships and locomotives. By means of these machines we can develop motive power to almost an indefinite extent at any place on the earth's surface, in deep mines and even on the middle of the ocean; while water and windmills are bound to special parts of the surface of the land. The locomotive transports travellers and goods over the land in numbers and with a speed which must have seemed an incredible fable to our forefathers, who looked upon the mailcoach with its six passengers in the inside, and its ten miles an hour, as an enormous progress. Steam engines traverse the ocean independently of the direction of the win, and, successfully resisting storms which would drive sailing vessels far away, reach their goal at the appointed time. The advantages which the concourse of numerous and variously skilled workmen in all branches offers in large towns where wind and water power are wanting, can be utilised, for steam engines find place everywhere, and supply the necessary crude force; thus the more intelligent human force may be spared for better purposes; and, indeed, wherever the nature of the ground or the neighbourhood of suitable lines of communication present a favourable opportunity for the development of industry, the motive power is also present in the form of steam-engines.
We see, then, that heat can produce mechanical power; but in the cases which we have discussed we have seen that the quantity of force which can be produced by a given measure of a physical process is always accurately defined, and that the further capacity for work of the natural forces is either diminished or exhausted by the work which has been performed. How is it now with Heat in this respect?
This question was of decisive importance in the endeavour to extend the law of the Conservation of Force to all natural processes. In the answer lay the chief difference between the older and newer views in these respects. Hence it is that many physicists designate that view of Nature corresponding to the law of the conservation of force with the name of Mechanical Theory of Heat.
The older view of the nature of heat was that it is a substance, very fine and imponderable indeed, but indestructible, and un-changeable in quantity, which is an essential fundamental property of all matter. And, in fact, in a large number of natural processes, the quantity of heat which can be demonstrated by the thermometer is unchangeable.
By conduction and radiation, it can indeed pass from hotter to colder bodies; but the quantity of heat which the former lose can be shown by the thermometer to have reappeared in the latter. Many processes, too, were known, especially in the passage of bodies from the solid to the liquid and gaseous states, in which heat disappeared—at any rate, as regards the thermometer. But when the gaseous body was restored to the liquid, and the liquid to the solid state, exactly the same quantity of heat reappeared which formerly seemed to have been lost. Heat was said to have become latent. On this view, liquid water differed from solid ice in containing a certain quantity of heat bound, which, just because it was bound, could not pass to the thermometer, and therefore was not indicated by it. Aqueous vapour contains a far greater quantity of heat thus bound. But if the vapour be precipitated, and the liquid water restored to the state of ice, exactly the same amount of heat is liberated as had become latent in the melting of the ice and in the vaporisation of the water.
Finally, heat is sometimes produced and sometimes disappears in chemical processes. But even here it might be assumed that the various chemical elements and chemical compounds contain certain constant quantities of latent heat, which, when they change their composition, are sometimes liberated and sometimes must be supplied from external sources. Accurate experiments have shown that the quantity of heat which is developed by a chemical process—for instance, in burning a pound of pure carbon into carbonic acid—is perfectly constant, whether the combustion is slow or rapid, whether it takes place all at once or by intermediate stages. This also agreed very well with the assumption, which was the basis of the theory of heat, that heat is a substance entirely unchangeable in quantity. The natural processes which have here been briefly mentioned, were the subject of extensive experimental and mathematical investigations, especially of the great French physicists in the last decade of the former, and the first decade of the present, century; and a rich and accurately-worked chapter of physics had been developed, in which everything agreed excellently with the hypothesis—that heat is a substance. On the other hand, the invariability in the quantity of heat in all these processes could at that time be explained in no other manner than that heat is a substance.
But one relation of heat—namely, that to mechanical work—had not been accurately investigated. A French engineer, Sadi Carnot, son of the celebrated War Minister of the Revolution, had indeed endeavoured to deduce the work which heat performs, by assuming that the hypothetical caloric endeavoured to expand like a gas; and from this assumption he deduced in fact a remarkable law as to the capacity of heat for work, which even now, though with an essential alteration introduced by Clausius, is among the bases of the modern mechanical theory of heat, and the practical conclusions from which, so far as they could at that time be compared with experiments, have held good.
But it was already known that whenever two bodies in motion rubbed against each other, heat was developed anew, and it could not be said whence it came.
The fact is universally recognised; the axle of a carriage which is badly greased and where the friction is great, becomes hot—so hot, indeed, that it may take fire; machine wheels with iron axles going at a great rate may become so hot that they weld to their sockets. A powerful degree of friction is not, indeed, necessary to disengage an appreciable degree of heat; thus, a lucifer match, which by rubbing is so heated that the phosphoric mass ignites, teaches this fact. Nay, it is enough to rub the dry hands together to feel the heat produced by friction, and which is far greater than the heating which takes place when the hands lie gently on each other. Uncivilized people use the friction of two pieces of wood to kindle a fire. With this view, a sharp spindle of hard wood is made to revolve rapidly on a base of soft wood in the manner represented in FIG. 99.
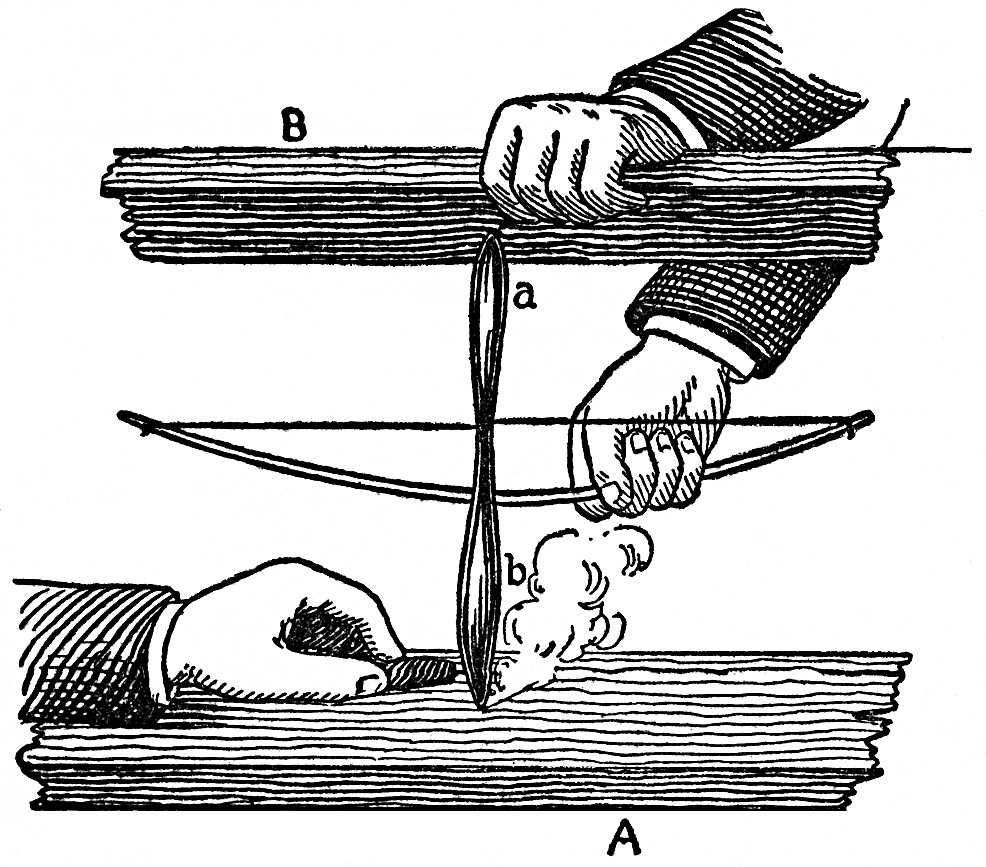
FIG. 99
So long as it was only a question of the friction of solids, in which particles from the surface become detached and compressed, it might be supposed that some changes in structure of the bodies rubbed might here liberate latent heat, which would thus appear as heat of friction.
But heat can also be produced by the friction of liquids, in which there could be no question of changes in structure, or of the liberation of latent heat. The first decisive experiment of this kind was made by Sir Humphry Davy in the commencement of the present century. In a cooled space he made two pieces of ice rub against each other, and thereby caused them to melt. The latent heat which the newly formed water must have here assimilated could not have been conducted to it by the cold ice, or have been produced by a change of structure; it could have come from no other cause than from friction, and must have been created by friction.
Heat can also be produced by the impact of imperfectly elastic bodies as well as by friction. This is the case, for instance, when we produce fire by striking flint against steel, or when an iron bar is worked for some time by powerful blows of the hammer.
If we inquire into the mechanical effects of friction and of inelastic impact, we find at once that these are the processes by which all terrestrial movements are brought to rest. A moving body whose motion was not retarded by any resisting force would continue to move to all eternity. The motions of the planets are an instance of this. This is apparently never the case with the motion of the terrestrial bodies, for they are always in contact with other bodies which are at rest, and rub against them. We can, indeed, very much diminish their friction, but never completely annul it. A wheel which turns about a well-worked axle, once set in motion, continues it for a long time; and the longer, the more truly and smoother the axle is made to turn, the better it is greased, and the less the pressure it has to support. Yet the vis viva of the motion which we have imparted to such a wheel when we started it, is gradually lost in consequence of friction. It disappears, and if we do not carefully consider the matter, it seems as if the vis viva which the wheel had possessed had been simply destroyed without any substitute.
A bullet which is rolled on a smooth horizontal surface continues to roll until its velocity is destroyed by friction on the path, caused by the very minute impacts on its little roughnesses.
A pendulum which as been put in vibration can continue to oscillate for hours if the suspension is good, without being driven by a weight; but by the friction against the surrounding air, and by that at its place of suspension, it ultimately comes to rest.
A stone which has fallen from a height has acquired a certain velocity on reaching the earth; this we know is the equivalent of a mechanical work; so long as this velocity continues as such, we can direct it upwards by means of suitable arrangements, and thus utilise it to raise the stone again. Ultimately the stone strikes against the earth and comes to rest; the impact has destroyed its velocity, and therewith apparently also the mechanical work which this velocity could have affected.
If we review the results of all these instances, which each of you could easily add to from your own daily experience, we shall see that friction and inelastic impact are processes in which mechanical work is destroyed, and heat produced in its place.
The experiments of Joule, which have been already mentioned, lead us a step further. He has measured in foot pounds the amount of work which is destroyed by the friction of solids and by the friction of liquids; and, on the other hand, he has determined the quantity of heat which is thereby produced, and has established a definite relation between the two. His experiments show that when heat is produced by the consumption of work, a definite quantity of work is required to produce that amount of heat which is known to physicists as the unit of heat; the heat, that is to say, which is necessary to raise one gramme of water through one degree centigrade. The quantity of work necessary for this is, according to Joule's best experiments, equal to the work which a gramme would perform in falling through a height of 425 metres.
In order to show how closely concordant are his numbers, I will adduce the results of a few series of experiments which he obtained after introducing the latest improvements in his methods.
1. A series of experiments in which water was heated by friction in a brass vessel. In the interior of this vessel a vertical axle provided with sixteen paddles was rotated, the eddies thus produced being broken by a series of projecting barriers, in which parts were cut out large enough for the paddles to pass through. The value of the equivalent was 424.9 metres.
2. Two similar experiments, in which mercury in an iron vessel was substituted for water in a brass one, gave 425 and 426.3 metres.
3. Two series of experiments, in which a conical ring rubbed against another, both surrounded by mercury, gave 426.7 and 425.6 metres.
Exactly the same relations between heat and work were also found in the reverse process—that is, when work was produced by heat. In order to execute this process under physical conditions that could be controlled as perfectly as possible, permanent gases and not vapours were used, although the latter are, in practice, more convenient for producing large quantities of work, as in the case of the steam engine. A gas which is allowed to expand with moderate velocity becomes cooled. Joule was the first to show the reason of this cooling. For the gas has, in expanding, to overcome the resistance which the pressure of the atmosphere and the slowly yielding side of the vessel oppose to it: or, if it cannot of itself overcome this resistance, it supports the arm of the observer which does it. Gas thus performs work, and this work is produced at the cost of its heat. Hence the cooling, If, on the contrary, the gas is suddenly allowed to issue into a perfectly exhausted space where it finds no resistance, it does not become cool, as Joule has shown; or if individual parts of it become cool, others become warm; and, after the temperature has become equalised, this is exactly as much as before the sudden expansion of the gaseous mass.
How much heat the various gases disengage when they are compressed, and how much work is necessary for their compression; or, conversely, how much heat disappears when they expand under a pressure equal to their own counterpressure, was partly known from the older physical experiments, and has partly been determined by the recent experiments of Regnault by extremely perfect methods. Calculations with the best data of this kind give us the value of the thermal equivalent from experiments:—
Withatmospheric air 426.0metres
“oxygen 425.7“
“nitrogen 431.3“
“hydrogen 425.3“
Comparing these numbers with those which determine the equivalence of heat and mechanical work in friction, as close an agreement is seen as can at all be expected from numbers which have been obtained by such varied investigations of different observers.
Thus then: a certain quantity of heat may be changed into a definite quantity of work; this quantity of work can also be retransformed into heat, and, indeed, into exactly the same quantity of heat as that from which it originated; in a mechanical point of view, they are exactly equivalent. Heat is a new form in which a quantity of work may appear.
These facts no longer permit us to regard heat as a substance, for its quantity is not unchangeable. It can be produced anew from the vis viva of motion destroyed; it can be destroyed, and then produces motion. We must rather conclude from this that heat itself is a motion, an internal invisible motion of the smallest elementary particles of bodies. If, therefore, motion seems lost in friction and impact, it is not actually lost, but only passes from the great visible masses to their smallest particles; while in steam engines the internal motion of the heated gaseous particles is transferred to the piston of the machine, accumulated in it, and combined in a resultant whole.
But what is the nature of this internal motion can only be asserted with any degree of probability in the case of gases. Their particles probably cross one another in rectilinear paths in all directions, until striking another particle, or against the side of the vessel, they are reflected in another direction. A gas would thus be analogous to a swarm of gnats, consisting, however, of particles infinitely small and infinitely more closely packed. This hypothesis, which has been developed by Krönig, Clausius, and Maxwell, very well accounts for all the phenomena of gases.
What appeared to the earlier physicists to be the constant quantity of heat is nothing more than the whole motive power of the motion of heat, which remains constant so long as it is not transformed into other forms of work, or results afresh from them.
We turn now to another kind of natural forces which can produce work—I mean the chemical. We have to-day already come across them. They are the ultimate cause of the work which gunpowder and the steam engine produce; for the heat which is consumed in the latter, for example, originates in the combustion of carbon—that is to say, in a chemical process. The burning of coal is the chemical union of carbon with the oxygen of the air, taking place under the influence of the chemical affinity of the two substances.
We may regard this force as an attractive force between the two, which, however, only acts through them with extraordinary power, if the smallest particles of the two substances are in closest proximity to each other. In combustion this force acts; the carbon and oxygen atoms strike against each other and adhere firmly, inasmuch as they form a new compound—carbonic acid—a gas known to all of you as that which ascends from all fermenting and fermented liquids—from beer and champagne. Now this attraction between the atoms of carbon and of oxygen performs work just as much as that which the earth in the form of gravity exerts upon a raised weight. When the weight falls to the ground, it produces an agitation, which is partly transmitted to the vicinity as sound waves, and partly remains as the motion of heat. The same result we must expect from chemical action. When carbon and oxygen atoms have rushed against each other, the newly-formed particles of carbonic acid must be in the most violent molecular motion—that is, in the motion of heat. And this is so. A pound of carbon burned with oxygen to form carbonic acid, gives as much heat as is necessary to raise 80.9 pounds of water from the freezing to the boiling point; and just as the same amount of work is produced when a weight falls, whether it falls slowly or fast, so also the same quantity of heat is produced by the combustion of carbon, whether this is slow or rapid, whether it takes place all at once, or by successive stages.
When the carbon is burned, we obtain in its stead, and in that of the oxygen, the gaseous product of combustion—carbonic acid. Immediately after combustion it is incandescent. When it has afterwards imparted heat to the vicinity, we have in the carbonic acid the entire quantity of carbon and the entire quantity of oxygen, and also the force of affinity quite as strong as before. But the action of the latter is now limited to holding the atoms of carbon and oxygen firmly united; they can no longer produce either heat or work any more than a fallen weight can do work if it has not been again raised by some extraneous force. When the carbon has been burnt we take no further trouble to retain the carbonic acid; it can do no more service, we endeavour to get it out of the chimneys of our houses as fast as we can.
Is it possible, then to tear asunder the particles of carbonic acid, and give to them once more the capacity of work which they had before they were combined, just as we can restore the potentiality of a weight by raising it from the ground? It is indeed possible. We shall afterwards see how it occurs in the life of plants; it can also be effected by inorganic processes, though in roundabout ways, the explanation of which would lead us too far from our present course.
This can, however, be easily and directly shown for another element, hydrogen, which can be burnt just like carbon. Hydrogen with carbon is a constituent of all combustible vegetable substances, among others, it is also an essential constituent of the gas which is used for lighting our streets and rooms; in the free state it is also a gas, the lightest of all, and burns when ignited with a feebly luminous blue flame. In this combustion—that is, in the chemical combination of hydrogen with oxygen, a very considerable quantity of heat is produced; for a given weight of hydrogen, four times as much heat as in the combustion of the same weight of carbon. The product of combustion is water, which, therefore, is not of itself further combustible for the hydrogen in it is completely saturated with oxygen. The force of affinity, therefore, of hydrogen for oxygen, like that of carbon for oxygen, performs work in combustion, which appears in the form of heat. In the water which has been formed during combustion, the force of affinity is exerted between the elements as before, but its capacity for work is lost. Hence the two elements must be again separated, their atoms torn apart, if new effects are to be produced from them.
This we can do by the aid of currents of electricity. In the apparatus depicted in FIG. 100, we have two glass vessels filled with acidulated water a and a1, which are separated in the middle by a porous plate moistened with water. In both sides are fitted platinum wires, k, which are attached to platinum plates, i and i1. As soon as a galvanic current is transmitted through the water by the platinum wires, k, you see bubbles of gas ascend from the plates i and i1. These bubbles are the two elements of water, hydrogen on the one hand, and oxygen of the other. The gases emerge through the tubes g and g1. If we wait until the upper part of the vessels and the tubes have been filled with it, we can inflame hydrogen at one side; it burns with a blue flame. If I bring a glimmering spill near the mouth of the other tube, it bursts into flame, just as happens with oxygen gas, in which the processes of combustion are far more intense than in atmospheric air, where the oxygen mixed with nitrogen is only one-fifth of the whole volume.
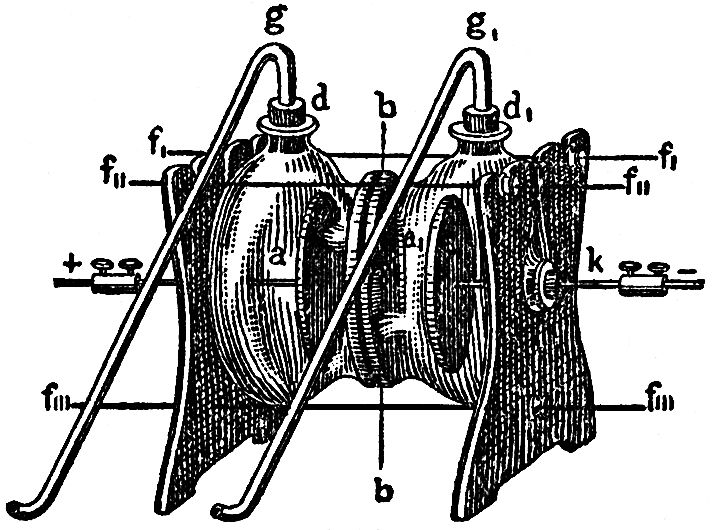
FIG. 100
If I hold a glass flask filled with water over the hydrogen flame, the water, newly formed in combustion, condenses upon it.
If a platinum wire be held in the almost non-luminous flame, you see how intensely it is ignited; in a plentiful current of a mixture of the gases, hydrogen and oxygen, which have been liberated in the above experiment, the almost infusible platinum might even be melted. The hydrogen which has here been liberated from the water by the electrical current has regained the capacity of producing large quantities of heat by a fresh combination with oxygen; its affinity for oxygen has regained for it its capacity for work.
We here become acquainted with a new source of work, the electric current which decomposes water. This current is itself produced by a galvanic battery, FIG. 101. Each of the four vessels contains nitric acid, in which there is a hollow cylinder of very compact carbon. In the middle of the carbon cylinder is a cylindrical porous vessel of white clay, which contains dilute sulphuric acid; in this dips a zinc cylinder. Each zinc cylinder is connected by a metal ring with the carbon cylinder of the next vessel, the last zinc cylinder, n, is connected with one platinum plate, and the first carbon cylinder, p, with the other platinum plate of the apparatus for the decomposition of water.
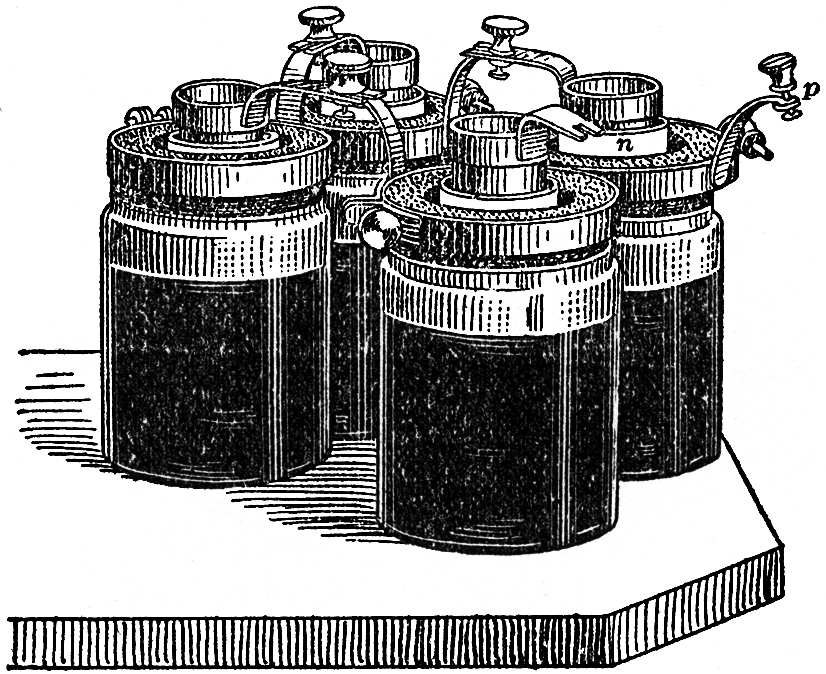
FIG. 101
If now the conducting circuit of this galvanic apparatus is completed, and the decomposition of water begins, a chemical process takes place simultaneously in the cells of the voltaic battery. Zinc takes oxygen from the surrounding water and undergoes a slow combustion. The product of combustion thereby produced, oxide of zinc, unites further with sulphuric acid, for which it has a powerful affinity, and sulphate of zinc, a saline kind of substance, dissolves in the liquid. The oxygen, moreover, which is withdrawn from it is taken by the water from the nitric acid surrounding the cylinder of carbon, which is very rich in it, and readily gives it up. Thus, in the galvanic battery, zinc burns to sulphate of zinc at the cost of the oxygen of nitric acid.
Thus, while one product of combustion, water, is again separated, a new combustion is taking place—that of zinc. While we there reproduce chemical affinity which is capable of work, it is here lost. The electrical current is, as it were, only the carrier which transfers the chemical force of the zinc uniting with oxygen and acid to water in the decomposing cell, and uses it for overcoming the chemical force of hydrogen and oxygen.
In this case, we can restore work which has been lost, but only by using another force, that of oxidising zinc.
Here we have overcome chemical forces by chemical forces, through the instrumentality of the electrical current. But we can attain the same object by mechanical forces, if we produce the electrical current by a magneto-electrical machine. If we turn the handle, the anker R R1, on which is coiled copper-wire, rotates in front of the poles of the horse-shoe magnet, and in these coils electrical currents are produced, which can be led from the points a and b. If the ends of these wires are connected with the apparatus for decomposing water, we obtain hydrogen and oxygen, though in far smaller quantity than by the aid of the battery which we used before. But this process is interesting, for the mechanical force of the arm which turns the wheel produces the work which is required for separating the combined chemical elements. Just as the steam engine changes chemical into mechanical force the magneto-electrical machine transforms mechanical force into chemical.
The application of electrical currents opens out a large number of relations between the various natural forces. We have decomposed water into its elements by such currents, and should be able to decompose a large number of other chemical compounds. On the other hand, in ordinary galvanic batteries electrical currents are produced by chemical forces.
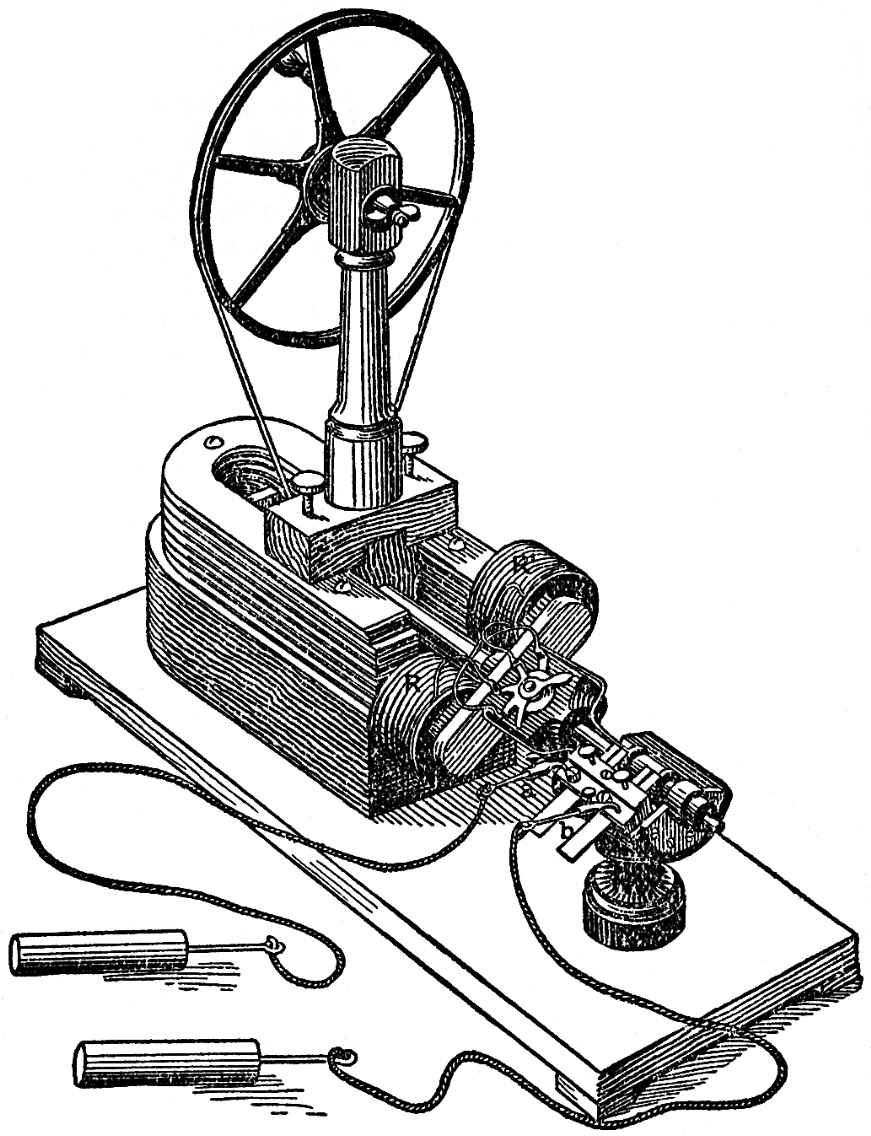
FIG. 102
In all conductors through which electrical currents pass they produce heat; I stretch a thin platinum wire between the ends n and p of the galvanic battery, FIG. 101; it becomes ignited and melts. On the other hand, electrical currents are produced by heat in what are called thermo-electric elements.
Iron which is brought near a spiral of copper wire, traversed by an electrical current, becomes magnetic, and then attracts other pieces of iron, or a suitably placed steel magnet. We thus obtain mechanical actions which meet with extended applications in the electrical telegraph, for instance. FIG. 103 represents a Morse's telegraph in one-third of the natural size. The essential part is a horse-shoe shaped iron core, which stands in the copper spirals b b. Just over the top of this is a small steel magnet c c, which is attracted the moment an electrical current, arriving by the telegraph wire, traverses the spirals b b. The magnet c c is rigidly fixed in the lever d d, at the other end of which is a style; this makes a mark on a paper band, drawn by a clock-work, as often and as long as c c is attracted by the magnetic action of the electrical current. Conversely, by reversing the magnetism in the iron core of the spirals b b, we should obtain in them an electrical current just as we have obtained such currents in the magneto-electrical machine, FIG. 102; in the spirals of that machine there is an iron core which, by being approached to the poles of the large horseshoe magnet, is sometimes magnetised in one and sometimes in the other direction.
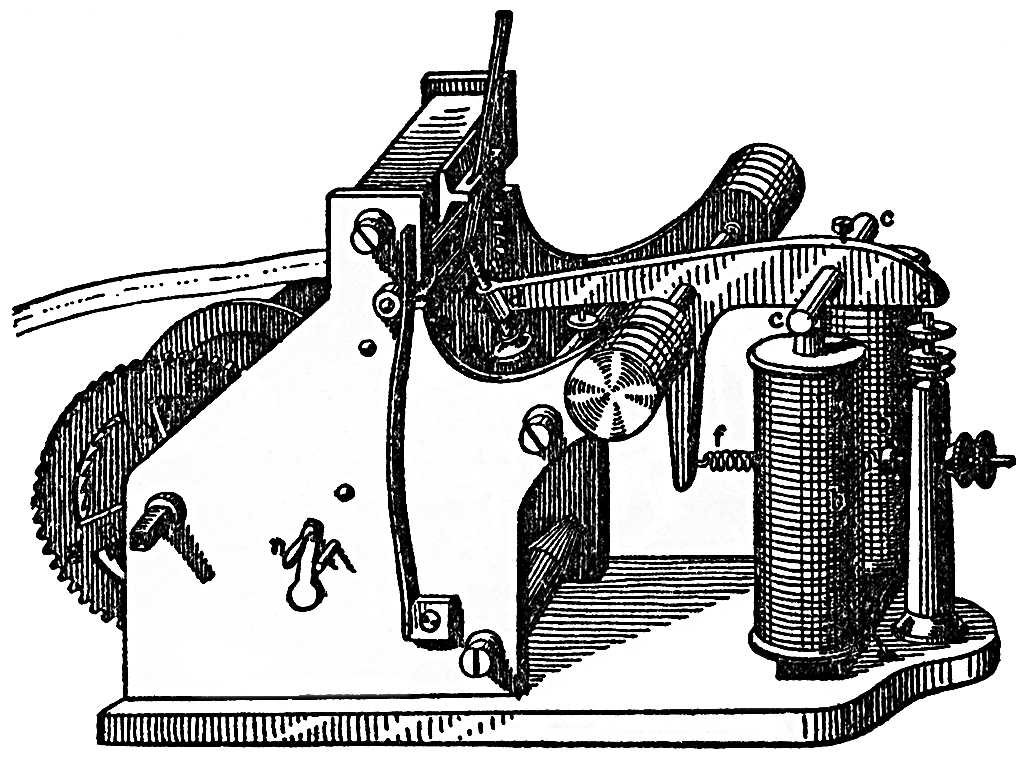
FIG. 103
I will not accumulate examples of such relations; in subsequent lectures we shall come across them. Let us review these examples once more, and recognise in them the law which is common to all.
A raised weight can produce work, but in doing so it must necessarily sink from its height, and, when it has fallen as deep as it can fall, its gravity remains as before, but it can no longer do work.
A stretched spring can do work, but in so doing it becomes loose. The velocity of a moving mass can do work, but in doing so it comes to rest. Heat can perform work; it is destroyed in the operation. Chemical forces can perform work, but they exhaust themselves in the effort.
Electrical currents can perform work, but to keep them up we must consume either chemical or mechanical forces, or heat.
We may express this generally. It is a universal character of all known natural forces that their capacity for work is exhausted in the degree in which they actually perform work.
We have seen, further, that when a weight fell without performing any work, it either acquired velocity or produced heat. We might also drive a magneto-electrical machine by a falling weight; it would then furnish electrical currents.
We have seen that chemical forces, when they come into play, produce either heat or electrical currents or mechanical work.
We have seen that heat may be changed into work; there are apparatus (thermo-electric batteries) in which electrical currents are produced by it. Heat can directly separate chemical compounds; thus, when we burn limestone, it separates carbonic acid from lime.
Thus, whenever the capacity for work of one natural force is destroyed, it is transformed into another kind of activity. Even within the circuit of inorganic natural forces, we can transform each of them into an active condition by the aid of any other natural force which is capable of work. The connections between the various natural forces which modern physics has revealed, are so extraordinarily numerous that several entirely different methods may be discovered for each of these problems.
I have stated how we are accustomed to measure mechanical work, and how the equivalent in work of heat may be found. The equivalent in work of chemical processes is again measured by the heat which they produce. By similar relations, the equivalent in work of the other natural forces may be expressed in terms of mechanical work.
If, now, a certain quantity of mechanical work is lost, there is obtained, as experiments made with the object of determining this point show, an equivalent quantity of heat, or, instead of this, of chemical force; and, conversely, when heat is lost, we gain an equivalent quantity of chemical or mechanical force; and, again, when chemical force disappears, an equivalent of heat or work; so that in all these interchanges between various inorganic natural forces working force may indeed disappear in one form, but then it reappears in exactly equivalent quantity in some other form; it is thus neither increased nor diminished, but always remains in exactly the same quantity. We shall subsequently see that the same law holds good also for processes in organic nature, so far as the facts have been tested.
It follows thence that the total quantity of all the forces capable of work in the whole universe remains eternal and unchanged throughout all their changes. All change in nature amounts to this, that force can change its form and locality without its quantity being changed. The universe possesses, once for all, a store of force which is not altered by any changed of phenomena, can neither be increased nor diminished, and which maintains any change which takes place on it.
You see how, starting from considerations based on the immediate practical interests of technical work, we have been led up to a universal natural law, which, as far as all previous experience extends, rules and embraces all natural processes; which is no longer restricted to the practical objects of human utility, but expresses a perfectly general and particularly characteristic property of all natural forces, and which, as regards generality, is to be placed by the side of the laws of the unalterability of mass, and the unalterability of the chemical elements.
At the same time, it also decides a great practical question which has been much discussed in the last two centuries, to the decision of which an infinity of experiments has been made and an infinity of apparatus constructed—that is, the question of the possibility of a perpetual motion. By this was understood a machine which was to work continuously without the aid of any external driving force. The solution of this problem promised enormous gains. Such a machine would have had all the advantages of steam without requiring the expenditure of fuel. Work is wealth. A machine which could produce work from nothing was as good as one which made gold. This problem had thus for a long time occupied the place of gold making, and had confused many a pondering brain. That a perpetual motion could not be produced by the aid of the then known mechanical forces could be demonstrated in the last century by the aid of the mathematical mechanics which had at that time been developed. But to show also that it is not possible even if heat, chemical forces, electricity, and magnetism were made to co-operate, could not be done without a knowledge of our law in all its generality. The possibility of a perpetual motion was first finally negatived by the law of the conservation of force, and this law might also be expressed in the practical form that no perpetual motion is possible, that force cannot be produced from nothing; something must be consumed.
You will only be ultimately able to estimate the importance and the scope of our law when you have before your eyes a series of its applications to individual processes in nature.
What I have to-day mentioned as to the origin of the moving forces which are at our disposal, directs us to something beyond the narrow confines of our laboratories and our manufactories, to the great operations at work in the life of the earth and of the universe. The force of falling water can only flow down from the hills when rain and snow bring it to them. To furnish these, we must have aqueous vapour in the atmosphere, which can only be effected by the aid of heat, and this heat comes from the sun. The steam engine needs the fuel which the vegetable life yields, whether it be the still active life of the surrounding vegetation, or the extinct life which has produced the immense coal deposits in the depths of the earth. The forces of man and animals must be restored by nourishment; all nourishment comes ultimately from the vegetable kingdom, and leads us back to the same source.
You see then that when we inquire into the origin of the moving forces which we take into our service we are thrown back upon the meteorological processes in the earth's atmosphere, on the life of plants in general, and on the sun.