LECTURE VI THE CORRELATION OF THE PHYSICAL FORCES
WE have frequently seen, during the course of these lectures, that one of those powers or forces of matter, of which I have written the names on that board, has produced results which are due to the action of some other force. Thus you have seen the force of electricity acting in other ways than in attracting; you have also seen it combine matters together or disunite them by means of its action on the chemical force; and in this case, therefore, you have an instance in which these two powers are related. But we have other and deeper relations than these; we have not merely to see how it is that one power affects another—how the force of heat affects chemical affinity, and so forth, but we must try and comprehend what relation they bear to each other, and how these powers may be changed one into the other; and it will to-day require all my care, and your care too, to make this clear to your minds. I shall be obliged to confine myself to one or two instances, because to take in the whole extent of this mutual relation and conversion of forces would surpass the human intellect.
In the first place, then, here is a piece of fine zinc foil, and if I cut it into narrow strips and apply to it the power of heat, admitting the contact of air at the same time, you will find that it burns; and then, seeing that it burns, you will be prepared to say that there is chemical action taking place. You see all I have to do is to hold the piece of zinc at the side of the flame, so as to let it get heated, and yet to allow the air which is flowing into the flame from all sides to have access to it; there is the piece of zinc burning just like a piece of wood, only brighter. A part of the zinc is going up into the air in the form of that white smoke, and part is falling down on to the table. This, then, is the action of chemical affinity exerted between the zinc and the oxygen of the air. I will show you what a curious kind of affinity this is by an experiment which is rather striking when seen for the first time. I have here some iron filings and gunpowder, and will mix them carefully together, with as little rough handling as possible; now we will compare the combustibility, so to speak, of the two. I will pour some spirit of wine into a basin and set it on fire; and, having our flame, I will drop this mixture of iron filings and gunpowder through it, so that both sets of particles will have an equal chance of burning. And now tell me which of them it is that burns? You see a plentiful combustion of the iron filings; but I want you to observe that, though they have equal chances of burning, we shall find that by far the greater part of the gunpowder remains untouched; I have only to drain off this spirit of wine, and let the powder which has gone through the flame dry, which it will do in a few minutes, and I will then test it with a lighted match. So ready is the iron to burn, that it takes, under certain circumstances, even less time to catch fire than gunpowder. [As soon as the gunpowder was dry, Mr. Anderson handed it to the lecturer, who applied a lighted match to it, when a sudden flash showed how large a proportion of gunpowder had escaped combustion when falling through the flame of alcohol.]
These are all cases of chemical affinity, and I show them to make you understand that we are about to enter upon the consideration of a strange kind of chemical affinity, and then to see how far we are enabled to convert this force of affinity into electricity or magnetism, or any other of the forces which we have discussed. Here is some zinc (I keep to the metal zinc, as it is very useful for our purpose), and I can produce hydrogen gas by putting the zinc and sulphuric acid together, as they are in that retort; there you see the mixture which gives us hydrogen—the zinc is pulling the water to pieces and setting free hydrogen gas. Now we have learned by experience that if a little mercury is spread over that zinc, it does not take away its power of decomposing the water, but modifies it most curiously. See how that mixture is now boiling; but when I add a little mercury to it the gas ceases to come off. We have now scarcely a bubble of hydrogen set free, so that the action is suspended for the time. We have not destroyed the power of chemical affinity, but modified it in a wonderful and beautiful manner. Here are some pieces of zinc covered with mercury exactly in the same way as the zinc in that retort is covered; and if I put this plate into sulphuric acid I get no gas, but this most extraordinary thing occurs, that if I introduce along with the zinc another metal which is not so combustible, then I reproduce all the action. I am now going to put to the amalgamated zinc in this retort some portions of copper wire (copper not being so combustible a metal as the zinc), and observe how I get hydrogen again, as in the first instance; there, the bubbles are coming over through the pneumatic trough, and ascending faster and faster in the jar; the zinc now is acting by reason of its contact with the copper.
Every step we are now taking brings us to a knowledge of new phenomena. That hydrogen which you now see coming off so abundantly does not come from the zinc, as it did before, but from the copper. Here is a jar containing a solution of copper. If I put a piece of this amalgamated zinc into it, and leave it there, it has scarcely any action; and here is a plate of platinum which I will immerse in the same solution, and might leave it there for hours, days, months, or even years, and no action would take place; but, by putting them both together, and allowing them to touch (FIG. 44), you see what a coating of copper there is immediately thrown down on the platinum. Why is this? The platinum has no power of itself to reduce that metal from that fluid, but it has, in some mysterious way, received this power by its contact with the metal zinc, Here, then, you see a strange transfer of chemical force from one metal to another; the chemical force from the zinc is transferred and made over to the platinum by the mere association of the two metals. I might take, instead of the platinum, a piece of copper or of silver, and it would have no action of its own on this solution, but the moment the zinc was introduced and touched the other metal, then the action would take place, and it would become covered with copper. Now is not this most wonderful and beautiful to see? We still have the identical chemical force of the particles of zinc acting, and yet, in some strange manner, we have power to make that chemical force, or something it produces, travel from one place to another; for we do make the chemical force travel from the zinc to the platinum by this very curious experiment of using the two metals in the same fluid in contact with each other.
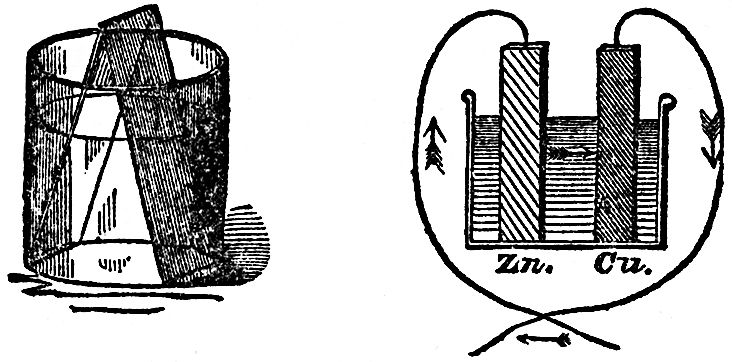
FIG. 44 FIG. 45
Let us now examine these phenomena a little more closely. Here is a drawing (FIG. 45) in which I have represented a vessel containing the acid liquid and the slips of zinc and platinum or copper, and I have shown them touching each other outside by means of a wire coming from each of them (for it matters not whether they touch in the fluid or outside; by pieces of metal attached, they still, by that communication between them, have this power transferred from one to the other). Now if, instead of only using one vessel, as I have shown there, I take another, and another, and put in zinc and platinum, zinc and platinum, zinc and platinum, and connect the platinum of one vessel with the zinc of another, the platinum of this vessel with the zinc of that, and so on, we should only be using a series of these vessels instead of one. This we have done in that arrangement which you see behind me. I am using what we call a Grove's voltaic battery, in which one metal is zinc and the other platinum; and I have as many as forty pairs of these plates all exercising their force at once in sending the whole amount of chemical power there evolved through these wires under the floor and up to these two rods coming through the table. We need do no more than just bring these two ends in contact, when the spark shows us what power is present; and what a strange thing it is to see that this force is brought away from the battery behind me, and carried along through these wires! I have here an apparatus (FIG. 46) which Sir Humphry Davy constructed many years ago, in order to see whether this power from the voltaic battery caused bodies to attract each other in the same manner as the ordinary electricity did. He made it in order to experiment with his large voltaic battery, which was the most powerful then in existence. You see there are in this glass jar two leaves of gold, which I can cause to move to and fro by this rack-work. I will connect each of these gold leaves with separate ends of this battery, and if I have a sufficient number of plates in the battery, I shall be able to show you that there will be some attraction between those leaves even before they come in contact; if I bring them sufficiently near when they are in communication with the ends of the battery, they will be drawn gently together; and you will know when this takes place, because the power will cause the gold leaves to burn away, which they could only do when they touched each other. Now I am going to cause these two leaves of gold to approach gradually, and I have no doubt that some of you will see that they approach before they burn, and those who are too far off to see them approach will see by their burning that they have come together. Now they are attracting each other, long before the connection is complete, and there they go! burnt up in that brilliant flash, so strong is the force. You thus see, from the attractive force at the two ends of this battery, that these are really and truly electrical phenomena.
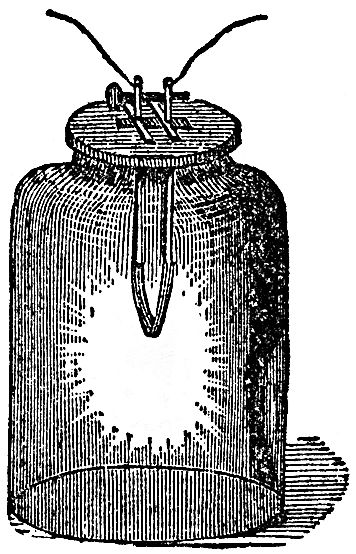
FIG. 46
Now let us consider what is this spark. I take these two ends and bring them together, and there I get this glorious spark like the sunlight in the heavens above us. What is this? It is the same thing which you saw when I discharged the large electrical machine, when you saw one single bright flash; it is the same thing, only continued, because here we have a more effective arrangement. Instead of having a machine which we are obliged to turn for a long time together, we have here a chemical power which sends forth the spark; and it is wonderful and beautiful to see how this spark is carried about through these wires. I want you to perceive, if possible, that this very spark and the heat it produces (for there is heat) is neither more nor less than the chemical force of the zinc—its very force carried along wires and conveyed to this place. I am about to take a portion of the zinc and burn it in oxygen gas for the sake of showing you the kind of light produced by the actual combustion in oxygen gas of some of this metal. [A tassel of zinc-foil was ignited at a spirit lamp and introduced into a jar of oxygen, when it burnt with a brilliant light.] That shows you what the affinity is when we come to consider it in its energy and power. And the zinc is being burned in the battery behind me at a much more rapid rate than you see in that jar, because the zinc is there dissolving and burning, and produces here this great electric light. That very same power which in that jar you saw evolved from the actual combustion of the zinc in oxygen, is carried along these wires and made evident here; and you may, if you please, consider that the zinc is burning in those cells, and that this is the light of that burning [bringing the two poles in contact and showing the electric light]; and we might so arrange our apparatus as to show that the amounts of power evolved in either case are identical. Having thus obtained power over the chemical force, how wonderfully we are able to convey it from place to place! When we use gunpowder for explosive purposes, we can send into the mine chemical affinity by means of this electricity; not having provided fire beforehand, we can send it in at the moment we require it. Now here (FIG. 47) is a vessel containing two charcoal points, and I bring it forward as an illustration of the wonderful power of conveying this force from place to place. I have merely to connect these by means of wires to the opposite ends of the battery, and bring the points in contact. See what an exhibition of force we have! We have exhausted the air so that the charcoal can not burn, and therefore the light you see is really the burning of the zinc in the cells behind me; there is no disappearance of the carbon, although we have that glorious electric light; and the moment I cut off the connection it stops. Here is a better instance to enable some of you to see the certainty with which we can convey this force, where under ordinary circumstances, chemical affinity would not act. We may absolutely take these two charcoal poles down under water, and get our electric light there. There they are in the water, and you observe, when I bring them into connection, we have the same light as we had in that glass vessel.
FIG. 47
Now besides this production of light, we have all the other effects and powers of burning zinc. I have a few wires here which are not combustible, and I am going to take one of them, a small platinum wire, and suspend it between these two rods which are connected with the battery, and when contact is made at the battery see what heat we get (FIG. 48). Is not that beautiful? It is a complete bridge of power. There is metallic connection all the way round in this arrangement, and where I have inserted the platinum, which offers some resistance to the passage of the force, you see what an amount of heat is evolved; this is the heat which the zinc would give if burnt in oxygen; but, as it is being burnt in the voltaic battery, it is giving it out at this spot. I will now shorten this wire for the sake of showing you that, the shorter the obstructing wire is, the more and more intense is the heat, until at last our platinum is fused and falls down, breaking off the circuit.
FIG. 48
Here is another instance. I will take a piece of the metal silver, and place it on charcoal connected with one end of the battery, and lower the other charcoal pole on to it. See how brilliantly it burns! (FIG. 49.) Here is a piece of iron on the charcoal: see what a combustion is going on; and we might go on in this way, burning almost every thing we place between the poles. Now I want to show you that this power is still chemical affinity; that if we call the power which is evolved at this point heat, or electricity,or any other name referring to its source, or the way in which it travels, we still shall find it to be chemical action. Here is a colored liquid which can show by its change of color the effects of chemical action; I will pour part of it into this glass, and you will find that these wires have a very strong action. I am not going to show you any effects of combustion or heat, but I will take these two platinum plates, and fasten one to the one pole and the other to the other end, and place them in this solution, and in a very short time you will see the blue color will be entirely destroyed. See, it is colorless now! I have merely brought the end of the wires into the solution of indigo, and the power of electricity has come through these wires and made itself evident by its chemical action. There is also another curious thing to be noticed now we are dealing with the chemistry of electricity, which is, that the chemical power which destroys the color is only due to the action on one side. I will pour some more of this sulphindigotic acid〖Sulphindigotic acid. A mixture of one part of indigo and fifteen parts of concentrated oil of vitriol. It is bleached on the side at which hydrogen gas is evolved in consequence of the liberated hydrogen withdrawing oxygen from the indigo, thereby forming a colorless deoxidized indigo. In making the experiment, only enough of the sulphindigotic acid must be added to give the water a decided blue color.〗 into a flat dish, and will then make a porous dike of sand separating the two portions of fluid into two parts (FIG. 50), and now we shall be able to see whether there is any difference in the two ends of the battery, and which it is that possesses this peculiar action. You see it is the one on my right hand which has the power of destroying the blue, for the portion on that side is thoroughly bleached, while nothing has apparently occurred on the other side. I say apparently, for you must not imagine that because you can not perceive any action none has taken place.
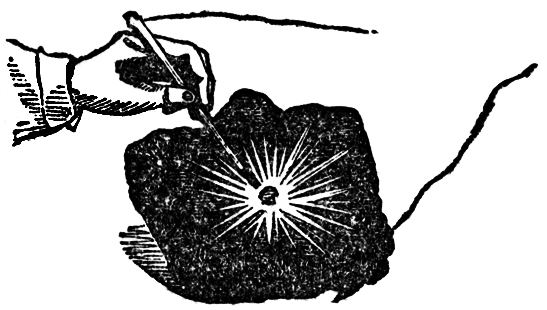
FIG. 49
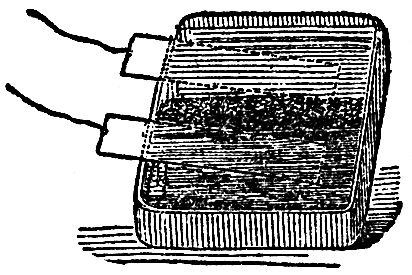
FIG. 50
Here we have another instance of chemical action. I take these platinum plates again and immerse them in this solution of copper, from which we formerly precipitated some of the metal, when the platinum and zinc were both put in it together. You see that these two platinum plates have no chemical action of any kind; they might remain in the solution as long as I liked, without having any power of themselves to reduce the copper; but the moment I bring the two poles of the battery in contact with them, the chemical action which is there transformed into electricity and carried along the wires again becomes chemical action at the two platinum poles, and now we shall have the power appearing on the left-hand side, and throwing down the copper in the metallic state on the platinum plate; and in this way I might give you many instances of the extraordinary way in which this chemical action or electricity may be carried about. That strange nugget of gold, of which there is a model in the other room, and which has an interest of its own in the natural history of gold, and which came from Ballarat, and was worth £8,000 or £9,000 when it was melted down last November, was brought together in the bowels of the earth, perhaps ages and ages ago, by some such power as this. And there is also another beautiful result dependent upon chemical affinity in that fine lead-tree〖Lead tree. To make a lead tree, pass a bundle of brass wires through the cork of a bottle, and fasten a plate of zinc round them just as they issue from the cork, so that the zinc may be in contact with every one of the wires. Make the wires to diverge so as to form a sort of cone, and, having filled the bottle quite full of a solution of sugar of lead, insert the wires and cork, and seal it down, so as to perfectly exclude the air. In a short time the metallic lead will begin to crystallize around the divergent wires, and form a beautiful object.〗, the lead growing and growing by virtue of this power. The lead and the zinc are combined together in a little voltaic arrangement in a manner far more important than the powerful one you see here, because in nature these minute actions are going on forever, and are of great and wonderful importance in the precipitation of metals and formation of mineral veins, and so forth. These actions are not for a limited time, like my battery here, but they act forever in small degrees, accumulating more and more of the results.
I have here given you all the illustrations that time will permit me to show you of chemical affinity producing electricity, and electricity again becoming chemical affinity. Let that suffice for the present; and now let us go a little deeper into the subject of this chemical force, or this electricity—which shall I name first?—the one producing the other in a variety of ways. These forces are also wonderful in their power of producing another of the forces we have been considering, namely, that of magnetism; and you know that it is only of late years, and long since I was born, that the discovery of the relations of these two forces of electricity and chemical affinity to produce magnetism has become known. Philosophers had been suspecting this affinity for a long time, and had long had great hopes of success; for in the pursuit of science we first start with hopes and expectations; these we realize and establish, never again to be lost, and upon them we found new expectations of farther discoveries, and so go on pursuing, realizing, establishing, and founding new hopes again and again.
Now observe this; here is a piece of wire which I am about to make into a bridge of force, that is to say, a communicator between the two ends of the battery. It is copper wire only, and is therefore not magnetic of itself. We will examine this wire with our magnetic needle (FIG. 51), and, though connected with one extreme end of the battery, you see that before the circuit is completed it has no power over the magnet. But observe it when I make contact; watch the needle; see how it is swung round; and notice how indifferent it becomes if I break contact again; so, you see, we have this wire evidently affecting the magnetic needle under these circumstances. Let me show you that a little more strongly. I have here a quantity of wire which has been wound into a spiral, and this will affect the magnetic needle in a very curious manner, because, owing to its shape, it will act very like a real magnet. The copper spiral has no power over that magnetic needle at present; but if I cause the electric current to circulate through it, by bringing the two ends of the battery in contact with the ends of the wire which forms the spiral, what will happen? Why, one end of the needle is most powerfully drawn to it; and if I take the other end of the needle, it is repelled; so, you see, I have produced exactly the same phenomena as I had with the bar magnet, one end attracting and the other repelling. Is not this, then, curious to see, that we can construct a magnet of copper? Furthermore, If I take an iron bar, and put it inside the coil, so long as there is no electric current circulating round, it has no attraction, as you will observe if I bring a little iron filings or nails near the iron. But now, if I make contact with the battery, they are attracted at once. It becomes at once a powerful magnet, so much so that I should not wonder if these magnetic needles on different parts of the table pointed to it. And I will show you, by another experiment, what an attraction it has. This piece and that piece of iron, and many other pieces, are now strongly attracted (FIG. 52); but, as soon as I break contact, the power is all gone, and they fall. What, then, can be a better or a stronger proof than this of the relation of the powers of magnetism and electricity? Again: here is a little piece of iron which is not magnetized. It will not, at present, take up any one of these nails; but I will take a piece of wire and coil it round the iron (the wire being covered with cotton in every part it does not touch the iron), so that the current must go round in this spiral coil; I am, in fact, preparing an electro-magnet (we are obliged to use such terms to express our meaning, because it is a magnet made by electricity—because we produce by the force of electricity a magnet of far greater power than a permanent steel one). It is now completed, and I will repeat the experiment which you saw the other day, of building up a bridge of iron nails: the contact is now made and the current is going through; it is now a powerful magnet; here are the iron nails which we had the other day, and now I have brought this magnet near them they are clinging so hard that I can scarcely move them with my hand (FIG. 53). But when the contact is broken, see how they fall. What can show you better than such an experiment as this the magnetic attraction with which we have endowed these portions of iron? Here, again, is a fine illustration of this strong power of magnetism. It is a magnet of the same sort as the one you have just seen. I am about to make the current of electricity pass through the wires which are round this iron for the purpose of showing you what powerful effects we get. Here are the poles of the magnet; and let us place an one of them this long bar of iron. You see, as soon as contact is made, how it rises in position (FIG. 54); and if I take such a piece as this cylinder, and place it on, woe be to me if I get my finger between; I can roll it over, but if I try to pull it off, I might lift up the whole magnet, but I have no power to overcome the magnetic power which is here evident. I might give you an infinity of illustrations of this high magnetic power. There is that long bar of iron held out, and I have no doubt that if I were to examine the other end I should find that it was a magnet. See what power it must have to support not only these nails, but all those lumps of iron hanging on to the end. What, then, can surpass these evidences of the change of chemical force into electricity, and electricity into magnetism? I might show you many other experiments whereby I could obtain electricity and chemical action, heat and light from a magnet, but what more need I show you to prove the universal correlation of the physical forces of matter, and their mutual conversion one into another?
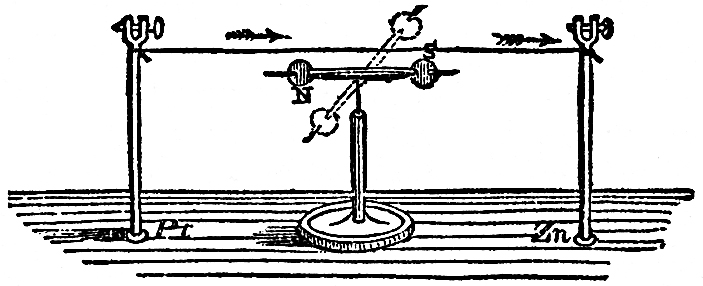
FIG. 51
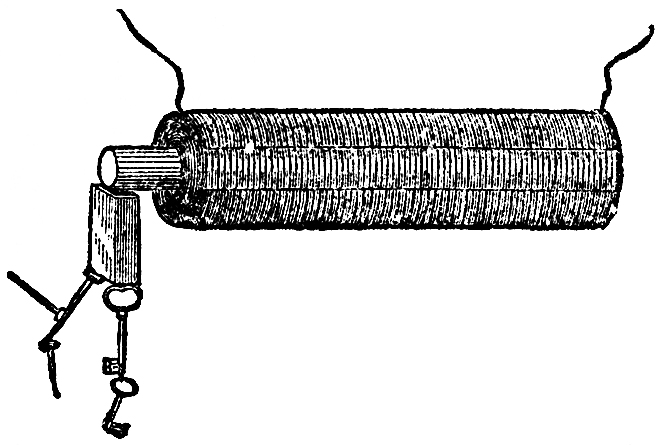
FIG. 52
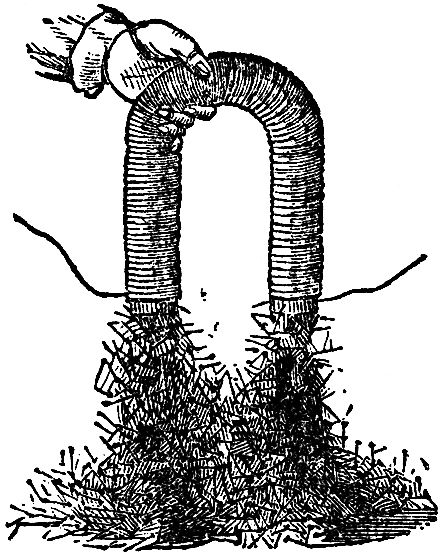
FIG. 53
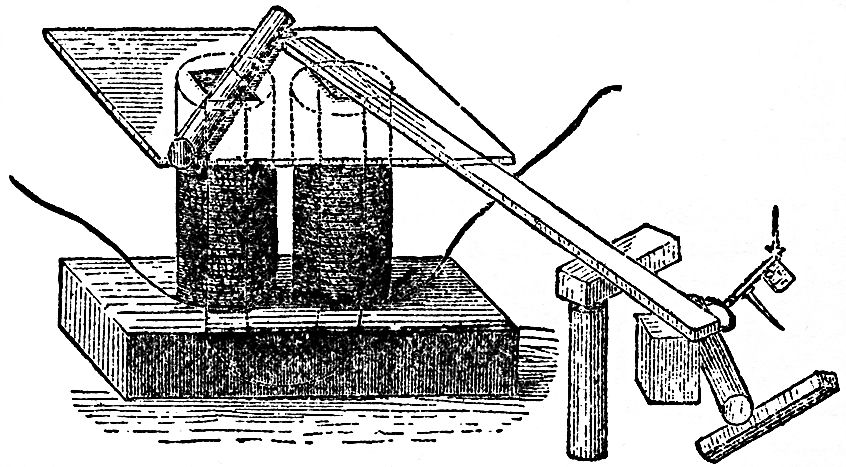
FIG. 54
And now let us give place as juveniles to the respect we owe to our elders, and for a time let me address myself to those of our seniors who have honored me with their presence during these lectures. I wish to claim this moment for the purpose of tendering our thanks to them, and my thanks to you all for the way in which you have borne the inconvenience that I at first subjected you to. I hope that the insight which you have here gained into some of the laws by which the universe is governed, may be the occasion of some among you turning your attention to these subjects; for what study is there more fitted to the mind of man than that of the physical sciences? And what is there more capable of giving him an insight into the actions of those laws, a knowledge of which gives interest to the most trifling phenomenon of nature, and makes the observing student find
“Tongues in trees, books in the running brooks,
Sermons in stones, and good in every thing?”